Virtual-Reality Guided Versus Fluoroscopy-Guided Transseptal Puncture in a Cardiac Phantom
Abstract: Objectives. We compared virtual-reality guided versus fluoroscopy-guided transseptal puncture by novice and experienced operators in a cardiac phantom. Outcome measures included accuracy, time, transseptal path distance, and a survey of the operator experience. Methods. A transseptal simulator was created using a Plexiglas case and a 3D-printed cardiac phantom with a replaceable fossa ovalis, a customized support, and an electromagnetic tracking system. A precisely registered virtual-reality rendering was constructed. To display the transseptal instruments in virtual reality, we attached electromagnetic sensors to standard transseptal instruments, including the needle, dilator, and sheath. Each subject completed 6 simulated transseptal punctures (3 fluoroscopy-guided and 3 virtual-reality guided). We measured the distance traversed by the transseptal needle, accuracy, and time for each simulated transseptal puncture. Operators were then surveyed regarding their experience. Results. A total of 8 subjects (6 faculty, 2 fellows) completed the trial. We found that virtual-reality guidance resulted in significantly more accurate puncture site selection and, subjectively, was more intuitive for the operator, particularly for novices. None of the participants experienced negative symptoms in virtual reality that required cessation of the procedure. Conclusions. Virtual reality compared with fluoroscopic guidance for transseptal puncture shows considerable promise, particularly for novice trainees, where it could lessen the learning curve. Current barriers to widespread implementation are discussed.
Key words: transseptal puncture, virtual reality
The transseptal puncture (TSP) is a minimally invasive endovascular procedure that allows operators to gain access into the left atrium (LA) from the systemic venous circulation for diagnostic and therapeutic procedures. Conventionally, it is performed under fluoroscopic guidance. However, the procedure is difficult to learn and, if performed incorrectly, can lead to significant adverse clinical consequences such as cardiac perforation, pericardial effusion, and tamponade. In addition, as the complexity of the interventional procedures that are being performed in the LA increase, it has become necessary to select the puncture site for a particular intervention with greater precision. When performed under 2-dimensional (2D) fluoroscopic guidance, the procedure requires a robust mental model of the 3-dimensional (3D) anatomy that takes considerable time and experience to develop. Recent advances in intracardiac echocardiography (ICE) have helped, but integrating this 2D information also requires considerable skill, and challenges exist in maintaining ultrasound visualization during the puncture itself. Head-mounted displays (HMDs) offer an alternative means of visualizing the operative field. Augmented reality (AR) is making headway into medical applications,1-4 but is limited in certain situations by its partially immersive experience. In contrast to the 2D planar nature of fluoroscopy and ICE, and the partially immersive nature of AR, virtual reality (VR) offers a fully immersive visualization that creates an intuitive 3D user-perspective.5 In this context, the user can be virtually placed inside a 3D rendering of a patient’s heart and navigate the TSP from this vantage point. We believe that this approach could ultimately shorten the learning curve and make the transseptal procedure safer. We hypothesized that VR-based transseptal guidance would subjectively be more intuitive and allow for greater confidence in choosing a puncture site, particularly for novice operators. In addition, we anticipated that VR guidance would reduce procedure time and improve the accuracy of the puncture site. The purpose of this single-center pilot study was to test these hypotheses in a simulated transseptal procedure using a 3D-printed cardiac phantom.
Methods
Subjects. Subjects consisted of Seattle Children’s Hospital Interventional Cardiac Catheterization and Electrophysiology faculty, as well as fellows from the University of Washington Pediatric Cardiology Training Program.
Protocol. The experiment was performed in the Electrophysiology Laboratory at Seattle Children’s Hospital. Study team research coordinators obtained informed consent. Subjects were then asked to complete a presurvey consisting of biographical data about the study participant. Following this, participants were instructed to pull a card from a bin to determine with which imaging modality (fluoroscopy or VR) they would begin. After donning a lead apron and vest, and with appropriate shielding in place, each subject performed 6 simulated TSP procedures, alternating between the 2 imaging modalities (3 fluoroscopic and 3 VR) (Figure 1). The research team took care to make sure the VR-HMD was properly fitted to the subject. All trials began with the needle-dilator-sheath combination positioned at the same place in the superior vena cava with the needle at the tip but not protruding from the sheath. Subjects were instructed to select a site for puncture as close to the center of the simulated fossa ovalis as possible and, once across the fossa ovalis, to advance the dilator and sheath such that the tip of the sheath was at the LA free wall near the origin of the left upper pulmonary vein. Both fellows were given basic instructions and provided a demonstration by one of the investigators (SPS) on how to perform TSP. During each trial, the following data points were collected:
- Time (in seconds) from start position to subject declaring they have selected a site for puncture.
- Accuracy (in millimeters) of the chosen site for puncture. The simulator recorded the location of each selected puncture site and computed the distance from the selected site and the true center of the simulated fossa ovalis.
- Total time (in seconds) to perform the TSP from beginning to end.
- Total distance (in millimeters) traveled by the needle from start to finish for each run.
After completing the 6 TSPs, subjects were asked to complete another survey.
Transseptal Simulation Platform
A 3D-printed heart model was generated using DICOM images obtained from a cardiac magnetic resonance imaging (MRI) performed on one of the research team members (SPS) in a 1.5 Tesla MRI scanner using standard clinical sequences. Segmentation of the DICOM images was performed using Slicer (www.slicer.org). The resultant STL file was further modified using Blender (www.blender.org) to include a slot between the right and left atria where we could insert a removable simulated fossa ovalis. The 3D heart model was then printed using a powder bed printer (Z Corporation). The removable simulated fossa ovalis consisted of a flexible plastic ring, approximately the size and shape of the fossa ovalis, onto which was affixed a piece of general-purpose office tape, which served as the simulated fossa ovalis (Figure 2A). The heart model was then placed in a custom made (www.blender.org) scaffold printed out of polylactic acid (PLA) using a Creator printer (Flashforge) to hold the heart in a fixed and appropriate orientation to mimic the cardiac position in a healthy supine adult human (Figure 2A). This scaffold was, in turn, fixed with Velcro tape to the floor of a plexiglass case the approximate size and shape of an adult human torso (Figure 2B). A piece of half-inch polyvinyl chloride tubing was used to simulate the vascular connection from the simulated thigh to the heart model’s junction between the inferior vena cava (IVC) and right atrium (RA). The simulated thigh consisted of a half circle of plexiglass with openings to simulate vascular access (Figure 2B). The TSP was performed using a Preface sheath and dilator (Biosense Webster) and a BRK transseptal needle (Abbott).
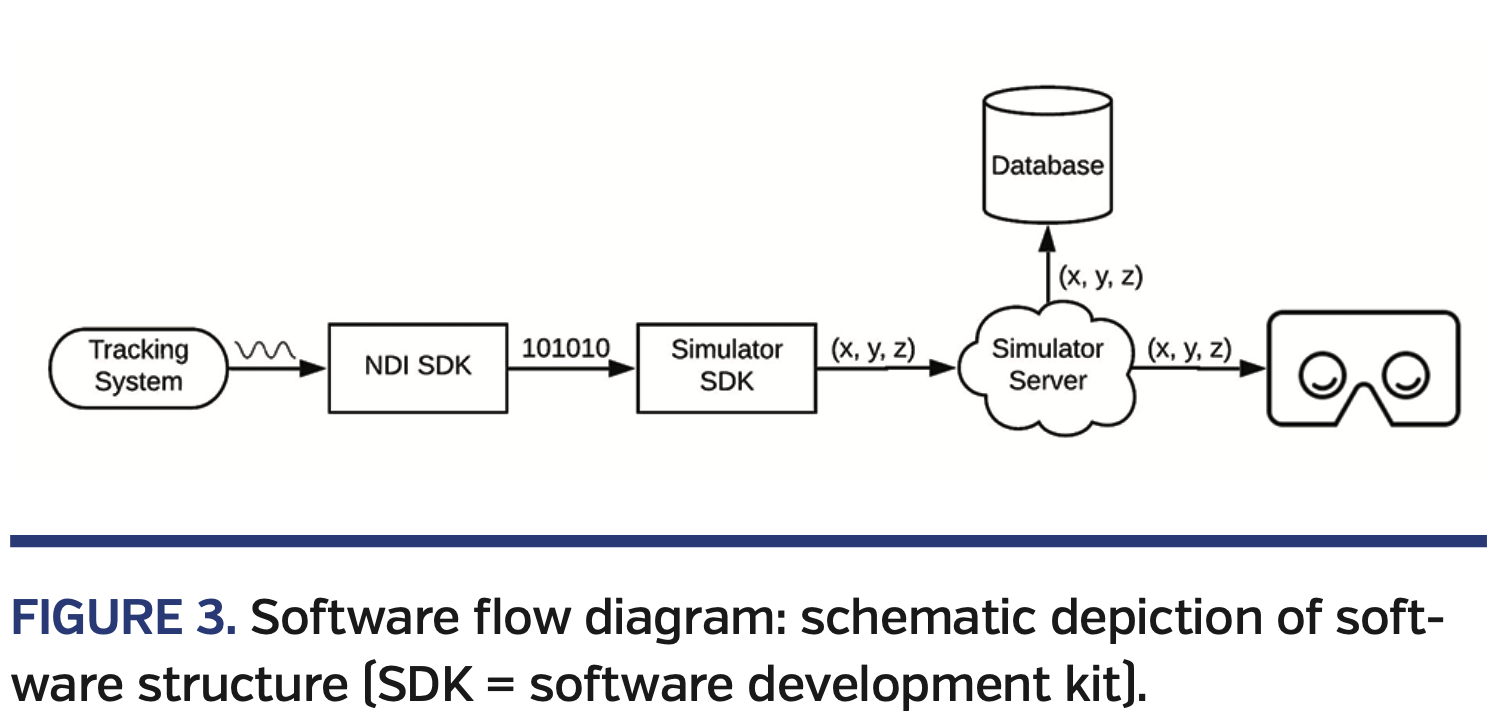
We used a commercially available electromagnetic (EM) tracking system (Northern Digital), with a 6 degrees-of-freedom sensor attached to each component of the transseptal equipment to display the dilator, sheath, and needle in VR. The transmitter portion of the EM tracking system was fixed to the floor of the plexiglasss case in a stable position relative to the cardiac model to allow for precise registration in the simulator’s coordinate system. We developed a series of software modules to transmit sensor coordinates from the tracking system to the headset via a proxy server (Figure 3). The first module used Northern Digital’s software development kit (SDK) to read sensor coordinates from the tracking system hardware every 10 ms (100 Hz). The coordinates were then translated to the common simulator coordinate system using our simulator’s SDK. Finally, the translated coordinates were pushed to the headset over a web socket connection hosted in the proxy server. All coordinates were acquired, translated, transmitted, and visualized without any perceived latency to the user.
Virtual Reality Set-Up
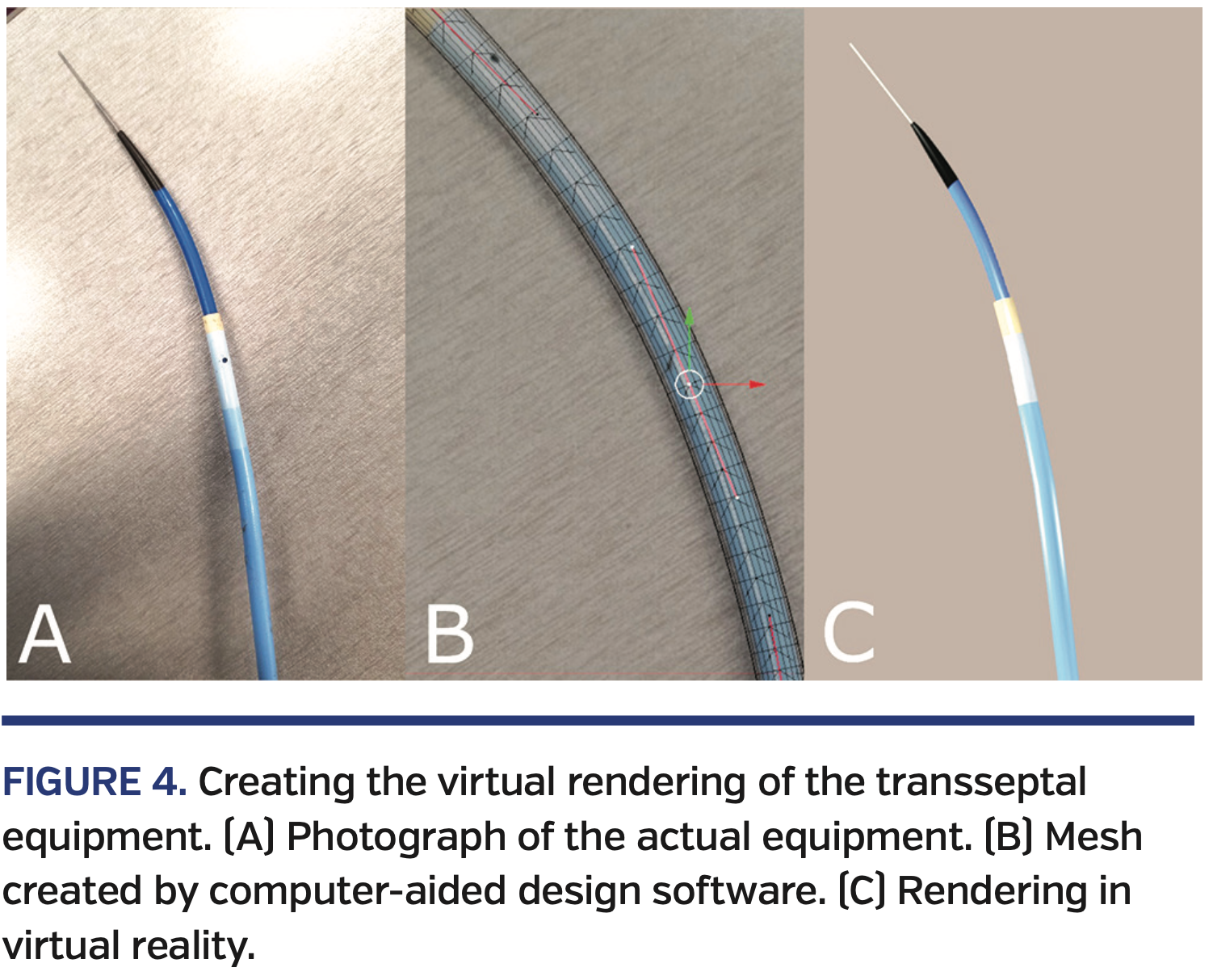
The visual components of the virtual reality environment consisted of the cardiac and scaffold models described above, in addition to models for the sheath, dilator, and needle. The sheath, dilator, and needle models were custom-created in Blender (www.blender.org) using the following process. First, top-down and side-view pictures were taken of each instrument (Figure 4A). Then, vertices, edges, and faces were aligned to the pictures in Blender to form a realistic mesh (Figure 4B). Finally, colors were added for visual effect (Figure 4C).
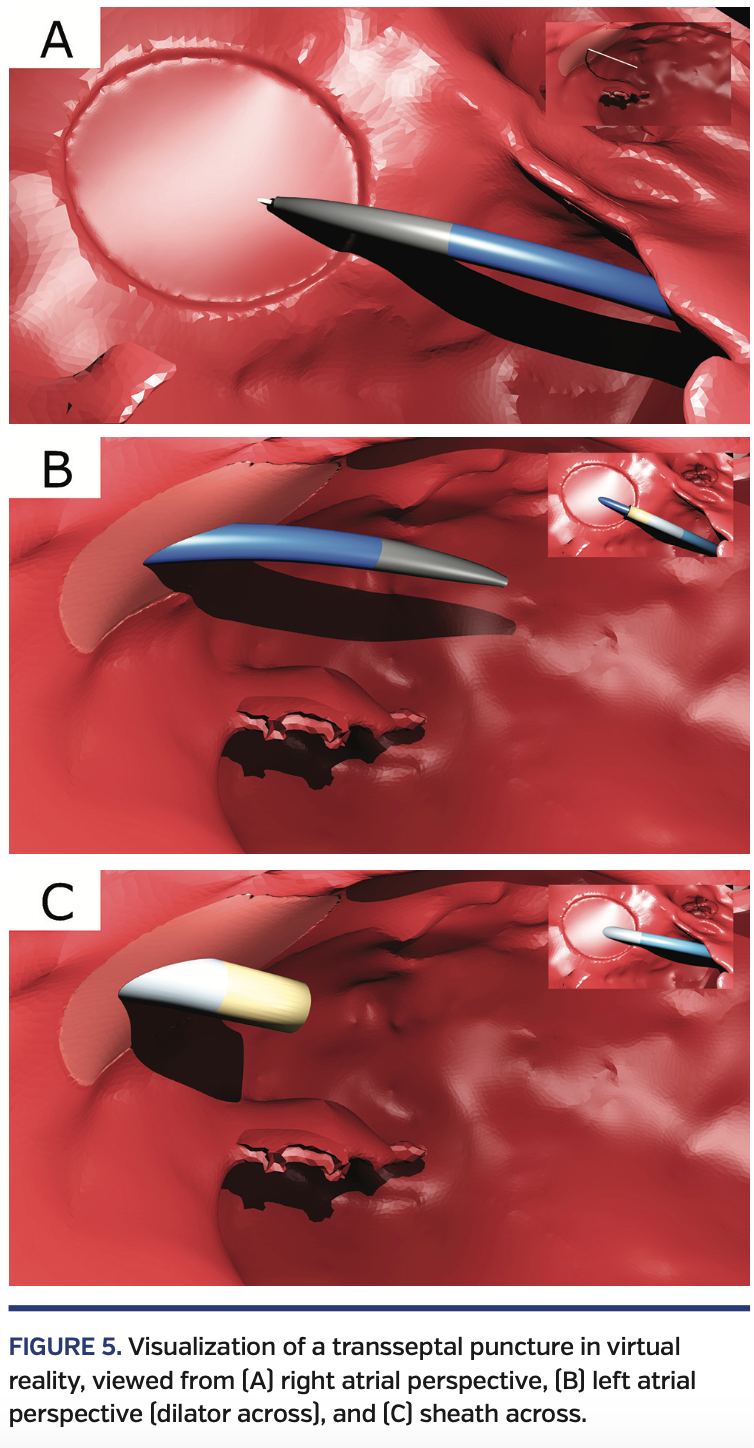
Each model (including the cardiac and scaffold models)was imported into Unity as an FBX file. We developed a collection of Unity-based C# scripts to stream in coordinates from the proxy server and adjusted the position and orientation of each model to reflect its physical counterpart. Finally, we developed two preset views. The first view was in the RA looking toward the septum (Figure 5A) and was primarily used to position the dilator and puncture the fossa.
The second view was near the mitral valve looking toward the LA side of the fossa ovalis (Figures 5B and 5C) and was primarily used to advance the sheath while retracting the dilator and needle. When in one view, the operator was given a “rear-view” camera image of the other view (Figures 5A-5C). Lights were placed in key locations on the virtual instruments and in the RA and LA to create shadows that facilitated guidance. A computer with an NVIDIA GTX 1080 graphics card was used to render the virtual environment at 60 frames/s. These frames were displayed in an Oculus Rift HMD (Oculus VR) that was attached to the computer.
Data Analysis
Summary statistics are presented as counts and percentages for categorical data and mean values and standard deviations for continuous data. Participants were randomly assigned to the first method of visualization and then alternated methods for a total of 3 per method per participant. Tests for differences between the methods of visualization were carried out using mixed linear-regression models with robust standard errors controlling for experience (attending vs fellow) and years of experience, with a random effect for the participant. Analyses and summaries were performed using Stata Statistical Software, version 15 (StataCorp LLC).
Results
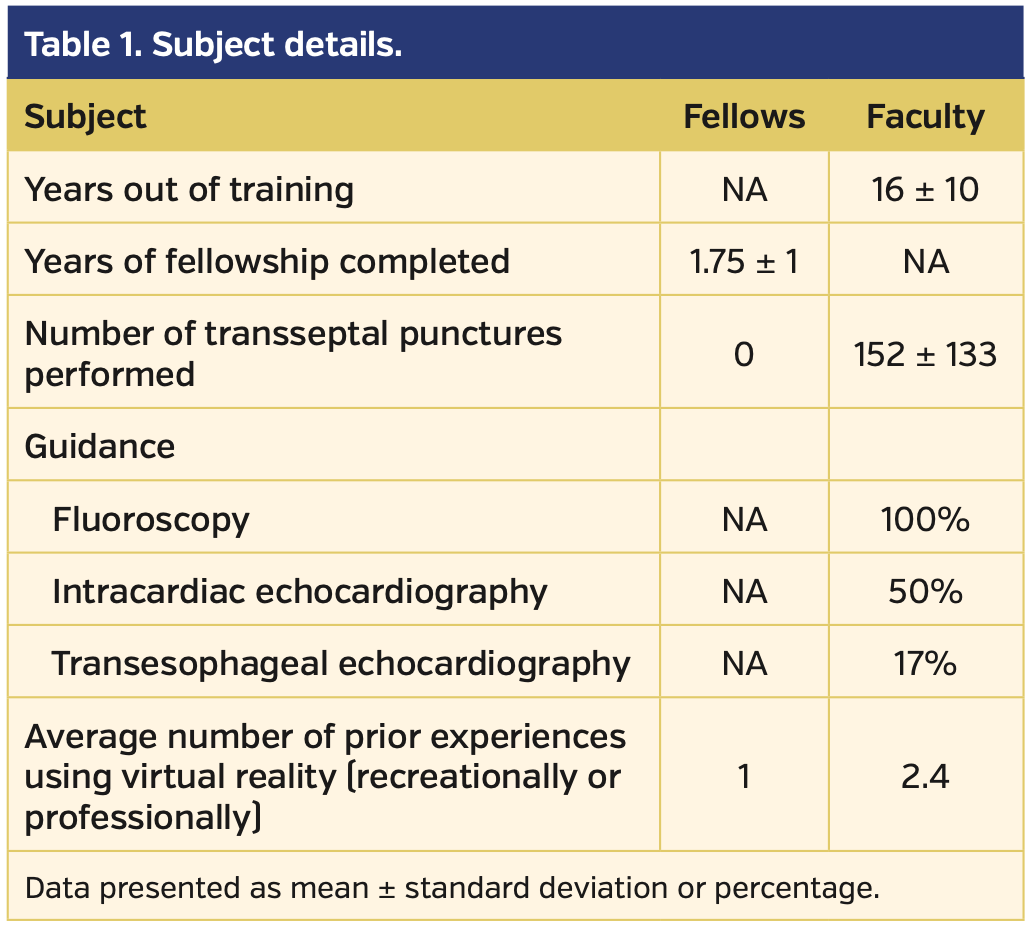
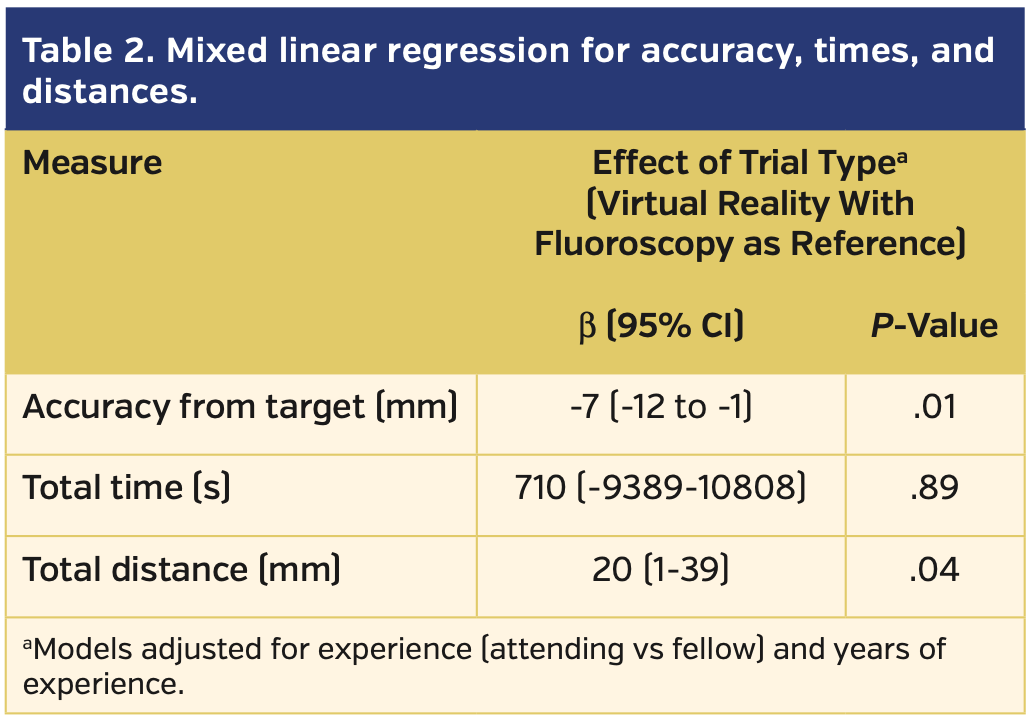
A total of 8 subjects (6 faculty and 2 fellows) completed the protocol. On average, the faculty subjects were highly experienced in TSP, while neither of the fellows had ever performed one. None of the subjects had significant prior experience in VR (Table 1). All transseptal attempts were completed successfully by all subjects regardless of whether fluoroscopy or VR was used for guidance. VR guidance was associated with significantly greater accuracy (P=.01), but a longer total distance traveled by the needle (mean, 22.5 cm vs 20.5 cm for fluoroscopy; P=.04) when compared with fluoroscopic guidance. Accuracy, measured as the mean distance from the puncture site to the true center of the simulated fossa, was 3.5 ± 3 mm using VR guidance vs 10 ± 10 mm using fluoroscopy. The times to puncture site selection and total times for TSP were not statistically different between the two groups (Table 2).
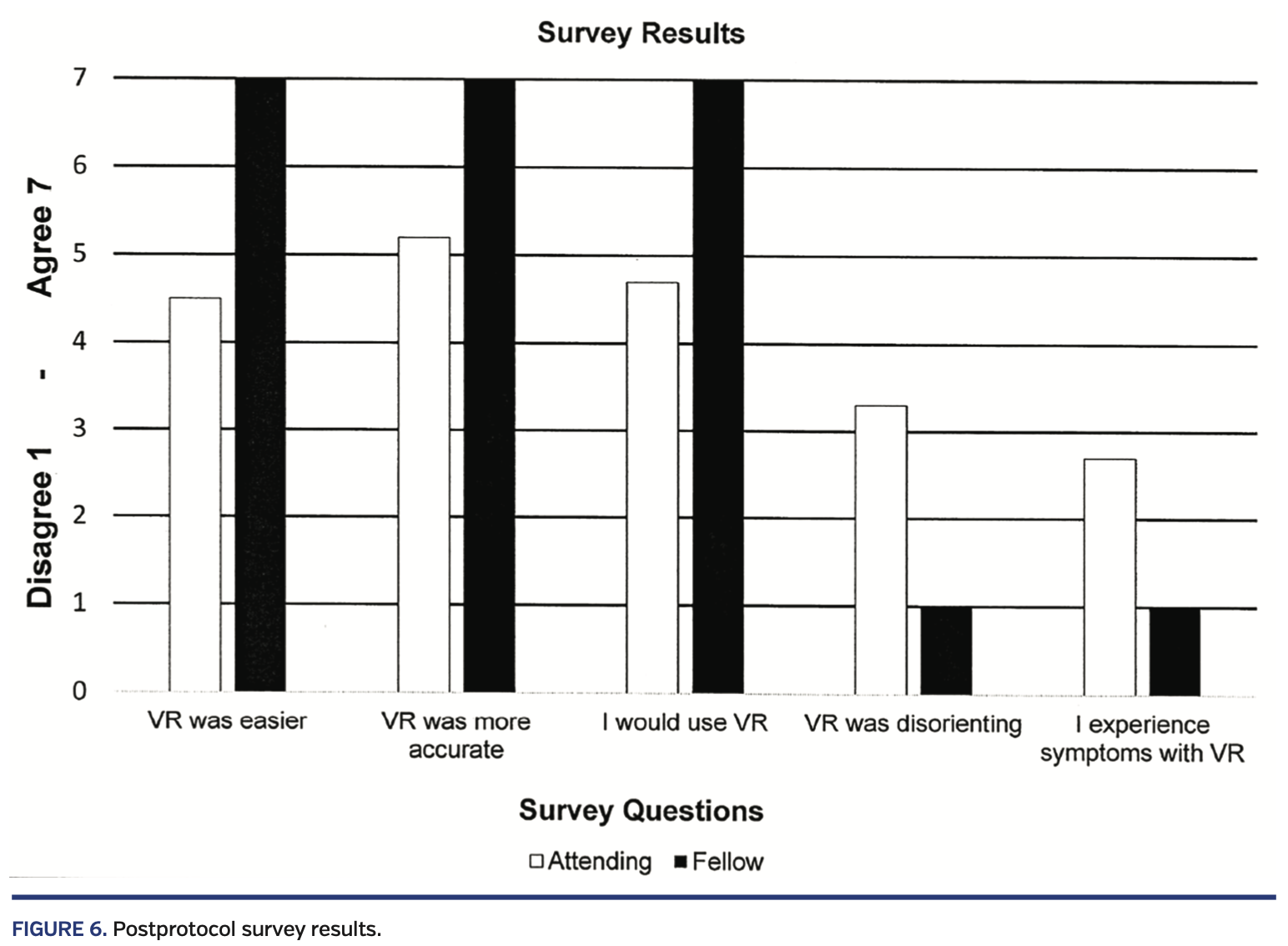
All subjects completed the post survey. On average, subjects rated VR guidance as “easier,” “more precise,” and “more efficient” than fluoroscopic guidance. No subject experienced disorientation or adverse symptoms requiring termination of the session. Though there were too few subjects for meaningful statistical analysis, fellows overall seemed to rate the VR experience higher than faculty (Figure 6).
Several subjects provided subjective comments. There were two comments related to the VR environment. One subject commented that it was uncomfortable when the sheath and dilator appeared to move “through” the wall of the virtual 3D model from their perspective. Another commented that they didn’t experience any adverse effects in VR. With respect to registration of the VR visualization to the physical model, one subject commented that they were not sure if the virtual image had “true” registration. Two others commented that VR was well registered with reality. Finally, one comment was related to the addition of new features, stating that color coding structures in the LA would be beneficial to positioning for interventions following a successful TSP.
Discussion
This study demonstrates proof-of-concept feasibility of VR guidance for TSP. Both experienced faculty and novice fellows performed TSPs more accurately when using VR relative to fluoroscopy. Furthermore, the majority of participants felt that navigating in VR was easier (more intuitive) than with fluoroscopy, and all but one agreed that they would use VR instead of fluoroscopy if it were approved for clinical use.
Not surprisingly, our study demonstrated that novice performers derived greater benefit from the VR experience as compared with experienced users. Having built a reliable and sophisticated mental model, experienced practitioners have less need for the intuitive clarity provided by VR. Even with this enhanced mental model, however, experienced providers were substantially more accurate in choosing the site for puncture using VR when compared with fluoroscopy. This has important implications for more complex LA interventional procedures such as LA appendage occlusion or mitral clip placement. For these procedures, where you cross the atrial septum significantly influences how well the procedure can be accomplished. In addition, for inexperienced users, training simulators that use VR might facilitate the development of a robust mental model and thus speed the learning curve, even for fluoroscopically guided procedures.
The advent of alternative imaging modalities, such as EM-based and impedance-based navigation systems and ultrasound, has decreased the use of fluoroscopy during catheter interventions, particularly for electrophysiology procedures.6 There are a number of published case series demonstrating the feasibility of entirely fluoroless procedures.7-10 However, despite this, fluoroscopy remains the imaging modality of choice during TSP by most interventional and electrophysiology providers.11,12 VR-HMDs have an inherent ability to make 3D information easier to understand and interact with, which may be an important catalyst for changing this paradigm. However, if we are to safely and effectively integrate VR HMDs into clinical workflows, we must first understand the implications of such a shift in practice.
Although the results of this study are quite promising, there are some important hurdles to overcome if we are going to implement this technology into clinical practice. Most notably, the near-universal implementation of non-fluoroscopic navigation systems in electrophysiology has not been realized in other endovascular fields, such as interventional cardiology and radiology. The development of such fluoroless navigation tools, tailored to the specific needs of these fields, will be necessary to overcome this barrier. The second barrier to widespread implementation of VR navigation during clinical cases is physician adoption. Although most of our subjects reported a positive experience, some found VR disorienting or nauseating. Although modern VR-HMDs have greatly improved compared with earlier models, there is still work to do for both hardware and software developers to make the experience comfortable, especially for longer use periods. In a previous study (unpublished), we found that some providers did not like the experience of being isolated from the operating room that VR produces. In this context, it is possible that augmented reality (AR) might be another valuable approach. Finally, for safety reasons, it is critically important that registration of the virtual world with the physical world be precise. In our simulated platform, we were able to achieve near-exact registration because we used a rigid non-beating model fixed to the floor of our simulation platform. The challenges of registering the elastic structure of a beating heart in real time, although clearly possible, will require careful execution and validation. We are presently developing technology to incorporate real-time ICE images into VR for this purpose.
Study limitations. Many operators now use ICE or transesophageal echocardiography as an adjunct to fluoroscopy. In our study, however, the technical challenges of making our simulation environment compatible with ultrasound were cost prohibitive. We acknowledge that the addition of ultrasound guidance in our study may have improved the performance of the fluoroscopy trials. Our study was also limited by a relatively small sample size, particularly for inexperienced providers. Finally, our highly controlled, rigid-model simulated platform also limits extrapolation to a beating heart scenario in a live clinical case, as discussed above. Nonetheless, the technical challenges to overcome to realize VR-guided TSP are readily achievable with currently available technology. What is needed is the collective will to move beyond what we are used to and embrace what may be a fundamentally superior visualization technology.
Conclusion
Our findings support the feasibility of VR-HMD guidance systems for the TSP procedure. We found that VR-guided TSP was more accurate and intuitive than TSP guided by fluoroscopy. For novice providers, VR guidance could potentially accelerate the learning curve. For experience providers, the enhanced accuracy could facilitate complex LA procedures. Further efforts should be aimed at breaking down the barriers to clinical implementation of this technology.
From the 1Department of Biomedical Informatics and Medical Education, University of Washington, Seattle, Washington; 2Division of Interventional Radiology, Department of Radiology, University of Washington, Seattle, Washington; 3Department of Biostatistics, University of Washington, Seattle, Washington; and the 4Division of Pediatric Cardiology, Department of Pediatrics, Seattle Children’s Hospital, University of Washington, Seattle, Washington.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Drs James and Seslar disclose stock-ownership and patent-licensing arrangements with Pyrus, Inc. The remaining authors report no conflicts of interest regarding the content herein.
Manuscript submitted July 22, 2019, provisional acceptance given July 24, 2019, final version accepted August 7, 2019.
Address for correspondence: Stephen P. Seslar, MD, PhD, Seattle Children’s Hospital, M/S RC.2.280, Seattle, WA 98105. Email: stephen.seslar@seattlechildrens.org
- Duong T, Wosik J, Christakopoulos GE, et al. Interpretation of coronary angiograms recorded using Google Glass: a comparative analysis. J Invasive Cardiol. 2015;27:443-446.
- Vaduganathan M, Bhatt DL, Heads up! Introducing wearable health technologies in cardiology. J Invasive Cardiol. 2015;27:447.
- Fuchs H, State A, Pisano ED, et al. Towards performing ultrasound-guided needle biopsies from within a head-mounted display. Vis Biomed Comput. 1996;1131:591-600.
- Bajura M, Fuchs H, Ohbuchi R. Merging virtual objects with the real world: seeing ultrasound imagery within the patient. Comput Graph (ACM). 1992;26:203-210.
- Held RT, Hui TT. A guide to stereoscopic 3D displays in medicine. Acad Radiol. 2011;18:1035-1048.
- Casella M, Dello Russo A, Pelargonio G, et al. Near zerO fluoroscopic exPosure during catheter ablAtion of supRavenTricular arrhYthmias: the NO-PARTY multicentre randomized trial. Europace. 2016;18:1565-1572.
- Ferguson JD, Helms A, Mangrum JM, et al. Catheter ablation of atrial fibrillation without fluoroscopy using intracardiac echocardiography and electroanatomic mapping. Circ Arrhythm Electrophysiol. 2009;2:611-619.
- Lyan E, Tsyganov A, Abdrahmanov A, et al. Non-fluoroscopic catheter ablation of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2018:1-24.
- Haegeli LM, Stutz L, Mohsen M, et al. Feasibility of zero or near zero fluoroscopy during catheter ablation procedures. Cardiol J. 2019;26:226-232.
- Liu X, Palmer J. Outcomes of 200 consecutive, fluoroless atrial fibrillation ablations using a new technique. Pacing Clin Electrophysiol. 2018;41:1404-1411.
- Sugrue A, Mbc B, Le KY, Dearani P, Asirvatham SJ. Minimizing radiation — why aren’t we down to zero? J Innov Card Rhythm Manag. 2018;9:3271-3273.
- Bunch TJ. Let go to grow: reconciling your dependence on fluoroscopy for atrial fibrillation ablations. Pacing Clin Electrophysiol. 2018;41:1412-1413.