Utilization of 3 Amplatzer Occluders for Closure of Post-Myocardial Infarction Ventricular Septal Defect
ABSTRACT: This case report describes a patient who sustained a post-myocardial infarction ventricular septal defect (VSD) with an associated left ventricular aneurysm who developed cardiogenic shock and required an intra-aortic balloon pump for hemodynamic stabilization. After deployment of a single Amplatzer occluder (AGA Medical), a residual VSD measuring 0.5 cm was noted. Therefore, a second Amplatzer occluder was deployed and a minimal residual VSD remained. The patient remained hemodynamically stable throughout the procedure and was subsequently extubated with removal of intra-aortic balloon pump. Post-discharge, the patient was readmitted with congestive heart failure. A third Amplatzer device was deployed to ameliorate the recurrent VSD shunt. At 9-week follow-up, transthoracic echocardiogram was performed and findings included: 1) left ventricular ejection fraction of 62%; 2) appearance of 3 Amplatzer devices along the interventrcular septum seated well with no motion and residual shunt; 3) moderate diastolic dysfunction with pseudonormal left ventricular filling pattern; and 4) no valvular abnormalities. The patient had increased exercise tolerance with no shortness of breath at rest or with exertion. This case demonstrates the utility and viability of multiple Amplatzer device deployment as a means of repairing a large post-myocardial infarction VSD and recurrent VSDs.
J INVASIVE CARDIOL 2012;24(5):E101-E103
Key words: Amplatzer occluder device, post-myocardial infarction ventricular septal defect, triple device deployment
______________________________________________
The development of post-myocardial infarction ventricular septal defects (VSD) is a known phenomenon, and the repair of VSDs with Amplatzer occluder devices (AGA Medical) is well documented.1,2,3 Post-myocardial infarction VSDs may cause significant hemodynamic compromise including cardiogenic shock and are typically treated with surgery or percutaneous closure.4 We report the first documented case in which 2 Amplatzer occluder devices were initially deployed in order to ameliorate a post-myocardial infarction VSD with a third occluder device subsequently deployed 8 weeks later.
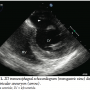 Case Report. A 52-year-old man with a history of diabetes mellitus, hypertension, and dyslipidemia was diagnosed with an acute inferior myocardial infarction at an outside hospital after he presented with weakness, dizziness, chest tightness, and worsening shortness of breath of 5 days duration. Physical examination revealed a moderate intensity pansystolic murmur at the left lower sternal border with radiation to the apex and base and clinical findings consistent with congestive heart failure. He had an elevated troponin I of 1.6 ng/mL and inferior ST elevations on electrocardiogram. The patient underwent a successful percutaneous coronary intervention to an occluded right coronary artery. Left ventriculography and transthoracic echocardiogram (TTE) was suggestive of a possible ventricular septal defect. An intra-aortic balloon pump was inserted in order to optimize hemodynamics, and the patient was transferred to our facility.
Case Report. A 52-year-old man with a history of diabetes mellitus, hypertension, and dyslipidemia was diagnosed with an acute inferior myocardial infarction at an outside hospital after he presented with weakness, dizziness, chest tightness, and worsening shortness of breath of 5 days duration. Physical examination revealed a moderate intensity pansystolic murmur at the left lower sternal border with radiation to the apex and base and clinical findings consistent with congestive heart failure. He had an elevated troponin I of 1.6 ng/mL and inferior ST elevations on electrocardiogram. The patient underwent a successful percutaneous coronary intervention to an occluded right coronary artery. Left ventriculography and transthoracic echocardiogram (TTE) was suggestive of a possible ventricular septal defect. An intra-aortic balloon pump was inserted in order to optimize hemodynamics, and the patient was transferred to our facility.
 Upon arrival to our hospital, TTE with color Doppler confirmed the suspected VSD, with a pulmonary to systemic flow ratio (Qp/Qs) of about 2.1:1 and a gradient of 64 mm Hg; left ventricular ejection fraction was normal. We decided to proceed with percutaneous closure of the VSD after he was deemed to be high-risk for surgical repair due to his recent myocardial infarction, obesity, and the size/location of the defect. Three days after admission, the patient was brought to the interventional cardiology suite for percutaneous closure of his VSD.
Upon arrival to our hospital, TTE with color Doppler confirmed the suspected VSD, with a pulmonary to systemic flow ratio (Qp/Qs) of about 2.1:1 and a gradient of 64 mm Hg; left ventricular ejection fraction was normal. We decided to proceed with percutaneous closure of the VSD after he was deemed to be high-risk for surgical repair due to his recent myocardial infarction, obesity, and the size/location of the defect. Three days after admission, the patient was brought to the interventional cardiology suite for percutaneous closure of his VSD.
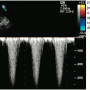 Under general anesthesia, intraoperative transesophageal echocardiogram (TEE) revealed the following pertinent findings: 1) left ventricular function with an ejection fraction of 55%; 2) large true inferior wall aneurysm of the left ventricle (Figure 1); 3) a basal-inferior VSD measuring approximately 1.1 cm in diameter with high velocity signal (Figures 2 and 3); 4) moderately dilated right ventricle with mildly reduced right ventricular function; and 5) moderate tricuspid regurgitation.
Under general anesthesia, intraoperative transesophageal echocardiogram (TEE) revealed the following pertinent findings: 1) left ventricular function with an ejection fraction of 55%; 2) large true inferior wall aneurysm of the left ventricle (Figure 1); 3) a basal-inferior VSD measuring approximately 1.1 cm in diameter with high velocity signal (Figures 2 and 3); 4) moderately dilated right ventricle with mildly reduced right ventricular function; and 5) moderate tricuspid regurgitation.
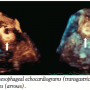 A 16 mm VSD Amplatzer occluder device (AGA Medical) was deployed across the VSD in the standard technique under fluoroscopic and echocardiographic (2D and 3D) guidance through a 9 Fr x 180 cm TorqVue delivery system (AGA Medical) placed in the right internal jugular vein. The first device was inserted via a left ventricular approach with release from the delivery cable and was anchored in the surrounding tissue. After deployment, TEE revealed significant residual shunting with color-flow disturbance and a VSD measuring 0.5 cm in
A 16 mm VSD Amplatzer occluder device (AGA Medical) was deployed across the VSD in the standard technique under fluoroscopic and echocardiographic (2D and 3D) guidance through a 9 Fr x 180 cm TorqVue delivery system (AGA Medical) placed in the right internal jugular vein. The first device was inserted via a left ventricular approach with release from the delivery cable and was anchored in the surrounding tissue. After deployment, TEE revealed significant residual shunting with color-flow disturbance and a VSD measuring 0.5 cm in 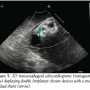 length in another echocardiographic plane of the interventricular septum. The VSD was determined to possess a unique serpiginous, S-shaped anatomy with adjacent aneurysm. We decided to deploy a second Amplatzer occluder (12 mm) in order to address the residual VSD. The second device was placed adjacent to the first occluder and appeared to be parallel when interrogated with 3D TEE (Figure 4). Color Doppler 2D TEE showed a very small residual VSD (Figure 5); left ventricular size was normal and left ventricular function was preserved.
length in another echocardiographic plane of the interventricular septum. The VSD was determined to possess a unique serpiginous, S-shaped anatomy with adjacent aneurysm. We decided to deploy a second Amplatzer occluder (12 mm) in order to address the residual VSD. The second device was placed adjacent to the first occluder and appeared to be parallel when interrogated with 3D TEE (Figure 4). Color Doppler 2D TEE showed a very small residual VSD (Figure 5); left ventricular size was normal and left ventricular function was preserved.
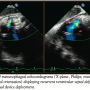 On postoperative day 1, the patient remained hemodynamically stable, and TTE revealed no change in residual VSD with well seated Amplatzer occluders absent of any rocking motion abnormalities; inferior left ventricular wall aneurysm was unchanged. On the second postoperative day, the patient was extubated and intra-aortic balloon pump was removed. The patient was discharged on postoperative day 8. At 30-day follow-up, the patient was clinically stable with no deterioration in status. However, approximately 8 weeks post-discharge, the patient presented to our facility with chief complaint of severe shortness of breath and evidence of congestive heart failure; TTE revealed recurrent VSD shunt (Figure 6). He was again deemed to be high-risk for surgical repair of VSD. The patient was readmitted and brought to the interventional cardiology suite for further evaluation. Under general anesthesia, TEE confirmed the presence of residual VSD with shunt at the basal septal wall. A 12 mm VSD Amplatzer occluder device was deployed across the VSD in the standard technique under fluoroscopic and echocardiographic guidance through a 9 Fr x 180 cm TorqVue delivery system placed in right internal jugular vein. Post-deployment, the third device was noted to have diminished the VSD. All 3 Amplatzer occluder devices were aligned parallel to one another (Figures 7A and 7B).
On postoperative day 1, the patient remained hemodynamically stable, and TTE revealed no change in residual VSD with well seated Amplatzer occluders absent of any rocking motion abnormalities; inferior left ventricular wall aneurysm was unchanged. On the second postoperative day, the patient was extubated and intra-aortic balloon pump was removed. The patient was discharged on postoperative day 8. At 30-day follow-up, the patient was clinically stable with no deterioration in status. However, approximately 8 weeks post-discharge, the patient presented to our facility with chief complaint of severe shortness of breath and evidence of congestive heart failure; TTE revealed recurrent VSD shunt (Figure 6). He was again deemed to be high-risk for surgical repair of VSD. The patient was readmitted and brought to the interventional cardiology suite for further evaluation. Under general anesthesia, TEE confirmed the presence of residual VSD with shunt at the basal septal wall. A 12 mm VSD Amplatzer occluder device was deployed across the VSD in the standard technique under fluoroscopic and echocardiographic guidance through a 9 Fr x 180 cm TorqVue delivery system placed in right internal jugular vein. Post-deployment, the third device was noted to have diminished the VSD. All 3 Amplatzer occluder devices were aligned parallel to one another (Figures 7A and 7B).
 At 9-week follow-up, TTE was performed and findings included: 1) left ventricular ejection fraction of 62%; 2) appearance of 3 Amplatzer devices along the interventricular septum seated well with no motion and residual shunt; 3) moderate diastolic dysfunction with pseudonormal left ventricular filling pattern; and 4) no valvular abnormalities. The patient had increased exercise tolerance with no shortness of breath at rest or with exertion.
At 9-week follow-up, TTE was performed and findings included: 1) left ventricular ejection fraction of 62%; 2) appearance of 3 Amplatzer devices along the interventricular septum seated well with no motion and residual shunt; 3) moderate diastolic dysfunction with pseudonormal left ventricular filling pattern; and 4) no valvular abnormalities. The patient had increased exercise tolerance with no shortness of breath at rest or with exertion.
Discussion. The deployment of single Amplatzer occluder devices has been established as a viable method of repairing post-myocardial infarction VSD. However, this case report demonstrates the first documented utilization of dual Amplatzer device deployment with subsequent insertion of a third Amplatzer device as a means of VSD closure with the use of real-time 2D and 3D TEE. This patient’s VSD anatomy was unique in that it possessed a serpiginous, S-shaped morphology; this morphology precluded deployment of an initial single larger occluder at the initial intervention. As a result of the S-shaped VSD anatomy, this allowed semi-parallel insertion of 3 Amplatzer occluders adjacent to one another without any overlapping and no additional flow disturbances. While the initial VSD was successfully ameliorated with 2 Amplatzer occluders, it is postulated that a recurrent VSD developed as previously ischemic myocardium surrounding the devices became necrotic and the defect enlarged. Although the utilization of multiple Amplatzer device deployment for closure of post-myocardial infarction VSDs is not common, we demonstrate that multiple device deployment is possible especially with the concurrent use of 3D TEE as an additional imaging modality to guide placement along with fluoroscopic assistance.
Acknowledgments. The authors would like to thank Dr. Takahiro Shiota for his technical assistance in 3D TEE image acquisition, and Dr. Collins Kwarteng and Dr. Lorraine Lubin for reviewing the manuscript.
References
- Holzer R, Balzer D, Amin Z, et al. Transcatheter closure of postinfarction ventricular septal defects using the new Amplatzer muscular VSD occluder: Results of a U.S. Registry. Catheter Cardiovasc Interv. 2004;61(2):196-201.
- Lee EM, Roberts DH, Walsh KP. Transcatheter closure of a residual postmyocardial infarction ventricular septal defect with the Amplatzer septal occluder. Heart. 1998;80(5); 522-524.
- Holzer R, de Giovanni J, Walsh KP, et al. Transcatheter closure of perimembranous ventricular septal defects using the Amplatzer membranous VSD occluder: immediate and midterm results of an international registry. Catheter Cardiovasc Interv. 2006;68(4):620-628.
- Maltais S, Ibrahim RE, Basmadjian AJ, et al. Postinfarction ventricular septal defects: towards a new treatment algorithm? Ann Thorac Surg. 2009;87(3):687-692.
______________________________________________
From the 1Heart Institutue at Cedars-Sinai Medical Center, and 2Department of Anesthesiology, Cedars-Sinai Medical Center, Los Angeles, California.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted November 4, 2011, provisional acceptance given January 4, 2012, final version accepted January 16, 2012.
Address correspondence to: Dr Saibal Kar, 8631 W. Third Street, Room 415E, East Tower, Los Angeles, CA, 90048. Email: karsk@cshs.org












