Usefulness of N-acetylcysteine or Ascorbic Acid Versus Placebo to Prevent Contrast-Induced Acute Kidney Injury in Patients Undergoing Elective Cardiac Catheterization: A Single-Center, Prospective, Randomized, Double-Blind, Placebo-Controlled Trial
Download a PDF of this article.
Abstract: Background. Contrast-induced acute kidney injury (CI-AKI) is a serious complication of procedures requiring contrast media associated with rising costs, prolonged hospitalization, and increased mortality. The aim of this study was to assess whether prophylactic administration of standard dosages of intravenous N-acetylcysteine or ascorbic acid reduce the incidence of CI-AKI in patients with chronic renal insufficiency undergoing elective cardiac catheterization. Methods. In a single-center, prospective, randomized, double-blind, placebo-controlled trial, the preventive effects of N-acetylcysteine and ascorbic acid were evaluated in 520 patients with chronically impaired renal function (serum creatinine ≥1.3 mg/dL) undergoing elective cardiac catheterization. The study drugs (600 mg N-acetylcysteine, 500 mg ascorbic acid, placebo) were administered intravenously twice (at 24 hours and 1 hour before the procedure). Serum creatinine, estimated glomerular filtration rate (eGFR) and serum urea were assessed at baseline and at 24 hours and 72 hours after contrast media exposure. CI-AKI was defined as a postangiographical increase in serum creatinine ≥0.5 mg/dL. Results. The incidence of CI-AKI was 27.6% in the N-acetylcysteine group (P=.20 vs placebo group) and in 24.5% in the ascorbic acid group (P=.11 vs placebo group). CI-AKI occurred in 32.1% of the placebo group. Conclusions. Standard doses of N-acetylcysteine and ascorbic acid did not prevent CI-AKI in patients at high risk undergoing cardiac catheterization with non-ionic, low-osmolality contrast agent.
J INVASIVE CARDIOL 2013;25(6):276-283
Key words: contrast-induced acute kidney injury, contrast media complications, N-acetylcysteine, ascorbic acid
____________________________________________________
The incidence of contrast-induced nephropathy (CIN) in the general population is low and has been calculated as less than 2%.1 Patients with increased risk of CIN include those with impaired renal function, advanced age, diabetes mellitus, heart insufficiency, proteinuria, use of high contrast media doses, concurrent nephrotoxic medication, and dehydration.2,3 The incidence of CIN in such patients is significantly higher, in the range of 12%-50%.4-6 The occurrence of CIN, even if transient, has been associated with a long-term increase in cardiovascular events.6-8 CIN is the third most frequent cause of in-hospital acute renal failure after decreased renal perfusion due to hypotension and postoperative renal insufficiency9 with a prevalence of 12%, resulting in prolonged hospitalization, increased mortality with an odds ratio (OR) of 5.5, and rising costs of health care.10-13 The in-hospital mortality rate of patients with CIN requiring dialysis can be as high as 40%, and their rate of 2-year survival is 19%.14,15
CIN is defined as acute deterioration of renal function after administration of iodinated contrast media in the absence of other causes. In clinical studies, it is traditionally defined as an increase in serum creatinine level of at least 0.5 mg/dL (44.2 µmol/dL) or by a relative increase of at least 25% above the baseline value within 48 hours of exposure to radiographic contrast.16,17
Although the protective effects of preprocedural hydration are the most effective means of preventing CIN, the resulting volume load of approximately 2 L/d is not without risk, especially for patients suffering from poor left ventricular function, adult respiratory distress syndrome, or decompensated liver.18,19
Thus, considerable efforts, such as the use of low- or iso-osmolal contrast agents,20-22 the administration of sodium bicarbonate,23,24 or early hemodialysis, have been made over the past few years to reduce the incidence of CIN with some degree of success.
One of these attempts was the administration of antioxidants. Based on the possible role of oxidative damage in the kidney following contrast administration, N-acetylcysteine as an antioxidant with the ability to scavenge a variety of oxygen-derived free radicals and improve endothelium-dependent vasodilation has been tested for the prevention of CIN in various scenarios, with orally standard and intravenously high-dose strategies25,26 showing contradictory results.5,25-39 Of note, oral N-acetylcysteine has a bioavailability of 10% only due to a high first-pass effect,40 and the results of a dose-dependent effect of intravenous N-acetylcysteine are inconsistent. Moreover, side effects after high-dose N-acetylcysteine were reported in 14.6% of patients.27
The efficacy of ascorbic acid, another antioxidant agent, was evaluated in animal studies.41,42 Randomized, placebo-controlled trials with high-dose ascorbic acid in patients with impaired renal function undergoing percutaneous coronary or peripheral procedures either confirmed43 or disproved these observations.24
There are a lack of randomized trials involving intravenous N-acetylcysteine and ascorbic acid in standard dosages initiated on the day before contrast media exposure. To address this issue, we conducted a randomized and controlled study to prospectively evaluate the effect of intravenous N-acetylcysteine or ascorbic acid in standard dosages for preventing CIN, compared with prehydration in patients with chronic renal insufficiency endangered by contrast nephrotoxicity, who were electively admitted for cardiac catheterization.
Methods
Trial design. The design was a single-center, prospective, randomized, double-blind, placebo-controlled study. Patients were randomly assigned to the treatment groups as part of their scheduled cardiac catheterization procedure. Block randomization was used to ensure that the treatment arms had approximately the same size at any time during the trial. According to a randomization list created by the Institute of Medical Statistics and Informatics via SAS V9 (SAS Institute), patients were randomly allocated to one of the three following treatment arms according to a ratio of 2:2:1 — (1) N-acetylcysteine plus conventional therapy; (2) placebo plus conventional therapy; or (3) ascorbic acid plus conventional therapy. No other preventive drug treatments were administered to the study patients. The investigators noted the administration of any potentially nephrotoxic medications. To ensure blinding at the hospital, the local pharmacists managed the preparation, dispensing, and accountability of all study medications, as per code assignment.
Study population. Patients age 18 years or older with stable baseline serum creatinine concentration of ≥1.3 mg/dL (114.9 µmol/L) scheduled for diagnostic or interventional cardiac catheterization at the Department of Cardiology, Clinic of Wetzlar, Germany, were considered for enrollment. Patients were excluded if serum creatinine measurements varied ≥0.3 mg/dL in the 7 days prior to angiography to reassure that the renal insufficiency had no reversible component. Further exclusion criteria were exposure to contrast agents or nephrotoxic medication (ie, non-steroidal antiinflammatory drugs, aminoglycoside, vancomycine) within the week prior to cardiac catheterization, renal transplant recipients, and patients who had plasmocytoma, oxalosis, nephrolithiasis, hyperthyroidism, unavailability of adequate time prior to angiography to perform the study procedures, or previously known insensitivity to N-acetylcysteine or ascorbic acid. Pregnant and breast feeding women, as well as those with child-bearing potential not using an approved method of contraception were also excluded. Informed written consent was obtained from all patients before starting any procedures and after explaining the aims, methods, anticipated benefits, and potential study hazards.
Study procedures. All patients were well hydrated before angiography. Fluid therapy consisted of intravenous hydration with 0.9% saline at a rate of 1.0 mL/kg body weight/hour controlled by an infusion pump for 12 hours prior to contrast media administration and continuing for 12 hours afterward. Modifications of the rate and duration of intravenous hydration were permitted, depending on the clinical status of the patient.
Infusions of all three study drugs were prepared by the local pharmacists, who were aware of the study drug. All other clinical staff, investigators, research personnel, patients, and families were blinded to the treatment assignments for the duration of the trial. To ensure blinding, the 600 mg N-acetylcysteine (ACC inject; Hexal AG), 500 mg ascorbic acid (Vitamin C 500; Wörwag Pharma GmbH & Co. KG), and placebo were mixed in identical 250 mL intravenous bags of 0.9% saline and were administered intravenously over the course of 30 minutes, at 24 hours and 1 hour before applying the contrast material.
Patients were observed and questioned regarding adverse events and were instructed to report any symptoms. If the clinical team considered an adverse event to be related to the study drug, the procedure was discontinued and, whether serious or not, the adverse event was reported. The study drug could be continued if, in the judgement of the investigator or attending physician, the adverse event had been treated, the condition had been reversed, and the event was not considered as a result of the study drug. All adverse events were recorded during a 3-day follow-up period.
Serum creatinine, estimated creatinine glomerular filtration rate (eGFR) applying the Modification of Diet in Renal Disease (MDRD) formula,44 and serum urea levels were measured 7 days prior to admission, at the time of admission, before contrast material administration, and 24 hours and 72 hours following contrast dye exposure. Blood samples were analyzed in a blinded fashion at the local hospital-based laboratory with consistent methodology.
Cardiac catheterization. Cardiac catheterization with coronary angiography and/or percutaneous coronary intervention were performed according to local standards using the femoral approach. The low-osmolal, non-ionic contrast agent Ultravist iopromide (Bayer Health Care) was used in all cases. Adjunctive therapy and the dose of contrast agent were left to the discretion of the interventional cardiologist.
Primary endpoint (clinical definition) and study aims. The primary endpoint was the development of CIN. CIN was defined as an absolute increase in serum creatinine concentration of ≥0.5 mg/dL (≥44.2 µmol/L)27,28 within 72 hours after contrast agent exposure.
The primary aim of the study was the comparison of the rate of CIN between the treatment group receiving N-acetylcysteine and the placebo group. The secondary aim was the comparison of the rate of CIN between the treatment group receiving ascorbic acid and the placebo group. Furthermore, the incidence of adverse clinical events was determined and compared between the three groups.
Sample size. Based on the rate in patients with impaired renal function undergoing coronary angiography as reported by Diaz-Sandoval et al4 and Durham et al,5 we assumed a CIN rate of 25%. Under this assumption, we calculated 200 patients in the N-acetylcysteine group and 200 patients in the placebo group for the primary endpoint in order to detect a decrease of at least 50% in the occurrence of CIN, with a one-sided alpha error of 0.025 and a power of 89%.
Due to the practicability of this monocenter study, for the second aim of the study, we had to restrict the study population to 500 patients. Consequently, assigning 100 patients to the ascorbic acid group, we could detect a risk reduction of maximal 56% with a power of 80% and a one-sided alpha error of 0.025. To account for a 4% non-evaluable and drop-out rate, we planned to enroll a total number of 520 patients.
Statistical analysis. Analysis was done for the full-analysis set (modified intention-to-treat principle, MITT) and the per-protocol set. The distributions of the categorical variables were expressed as percentages and compared between the treatment arms by Fisher’s exact test. The distributions of the continuous variables were described by median with interquartile range (IQR) and standard deviation (SD), and compared with the Mann-Whitney U-test or Kruskal-Wallis H-test, as appropriate. The changes of serum creatinine, serum urea, and eGFR from baseline to 72 hours after contrast administration were illustrated by Box and Whisker plots.
Diabetes mellitus was prespecified for subgroup analysis. The Cochran-Mantel-Haenszel Test was used to test the influence of treatment (for instance, ascorbic acid and placebo) according to the occurrence of diabetes mellitus. Homogeneity of ORs was calculated with the Breslow-Day test. Sensitivity analysis was used to determine the effect of missing data. The statistical analysis was performed using SAS V9 and StatXact software.
To examine the effects of different confounding variables on the incidence of CIN, logistic regression was performed with the primary endpoint of CIN as the dependent variable (binary outcome). Treatment as independent variable, the amount of contrast dye (quartiles), and the baseline serum creatinine value (as dichotomous variable according to the median value ≤1.4 mg/dL and >1.4 mg/dL) as covariates were entered into the model. The Wald test was used to evaluate the strength of the treatment. Values of P ≤.05 were considered to be statistically significant in testing the primary aim of the study.
Eligibility for the per protocol set was defined to include patients who received all drug infusions, underwent cardiac catheterization during the study period, and had measurements of renal function at baseline and at 24 and 72 hours after angiography, and did not show protocol violations. Protocol violations were defined as failure to meet inclusion criteria, meeting exclusion criteria, multiple angiographic procedures during the study period, no cardiac catheterization after randomization, forbidden comedication, withdrawal of informed consent, incomplete laboratory measurements, and/or a serious clinical event during the study period.
Trial management. No interim analysis was performed. All adverse events were adjudicated and classified by the event-adjudication committee blinded to treatment assignment. The study was conducted in accordance with the ethical principles of Good Clinical Practice (GCP), the Declaration of Helsinki (Finland, 1964) including all subsequent amendments, and local regulatory requirements. The study was approved by the Ethics Committee of the Landesärztekammer Hessen in Frankfurt, Germany (No. 65/2004), and supervised by the Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) in Bonn, Germany (No. 4022894). All patients signed the written informed consent form after receiving oral and written information about the trial.
The design, conduct, interpretation, and analysis of the trial was not funded by the industry or other external sources, including grants. Funding was derived entirely from internal sources of the Clinic of Wetzlar. The authors designed and supervised the trial and the statistical analysis plan. The first author wrote the first draft of the manuscript. Subsequent drafts were prepared by the other authors. All authors attest that the study was performed in accordance with the protocol and the statistical analysis plan and vouch for the accuracy and completeness of the reported analysis.
Results
Study population. Between December 2004 and April 2008, a total of 520 patients were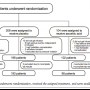 enrolled. Of these, 208 patients were assigned to the N-acetylcysteine group, 104 patients to the ascorbic acid group, and 208 patients to the placebo group. All 520 patients received at least one drug infusion and were therefore included in the safety set. Twenty-one patients dropped out due to distinct reasons (Figure 1). Thus, the full analysis set (MITT) contained 499 patients (N-acetylcysteine, n = 199; placebo, n = 198; ascorbic acid, n = 102). Sixteen patients had relevant protocol violations (Figure 1). Therefore, 483 patients were included in the per-protocol analysis (N-acetylcysteine, n = 192; placebo, n = 193; ascorbic acid, n = 98).
enrolled. Of these, 208 patients were assigned to the N-acetylcysteine group, 104 patients to the ascorbic acid group, and 208 patients to the placebo group. All 520 patients received at least one drug infusion and were therefore included in the safety set. Twenty-one patients dropped out due to distinct reasons (Figure 1). Thus, the full analysis set (MITT) contained 499 patients (N-acetylcysteine, n = 199; placebo, n = 198; ascorbic acid, n = 102). Sixteen patients had relevant protocol violations (Figure 1). Therefore, 483 patients were included in the per-protocol analysis (N-acetylcysteine, n = 192; placebo, n = 193; ascorbic acid, n = 98).
The baseline characteristics of the patients in the full-analysis set are expressed in Table 1. The groups were homogenous regarding age, gender, and body mass index. Cardiovascular risk factors were similar in all groups, although there was a small P-value computed for the comparison of the incidence of hypercholesterolemia (P=.031). All treatment groups appeared to be comparable in terms of previous myocardial infarction, coronary artery bypass grafting (CABG), or percutaneous coronary intervention (PCI), incidence of diabetic nephropathy and history of acute or contrast-media induced renal failure. The amount of intravenous volume applied pre- and postprocedure was not statistically different between the treatment groups.
There were no significant differences in the median contrast medium volume used during the procedure between treatment groups. Median contrast volume was 110 mL (IQR, 80-160 mL) in the N-acetylcysteine group, 115 mL (IQR, 90-150 mL) in the ascorbic acid group, and 110 mL (IQR, 80-150 mL) in the placebo group (P=.36).
procedure between treatment groups. Median contrast volume was 110 mL (IQR, 80-160 mL) in the N-acetylcysteine group, 115 mL (IQR, 90-150 mL) in the ascorbic acid group, and 110 mL (IQR, 80-150 mL) in the placebo group (P=.36).
Endpoint. The CIN rate was 27.6% (53/192) in the N-acetylcysteine group (95% CI, 21.4%-34.5%), 32.1% (62/193) in the placebo group (95% CI; 25.6%-39.2%), and 24.5% (24/98) in the ascorbic acid group (95% CI, 16.4%-34.2%). Concerning the primary aim of the study, there were no significant differences in CIN between the N-acetylcysteine group and the placebo group (P=.20). Also, there were no differences detected in the CIN rate between the ascorbic acid group and the placebo group in the secondary aim of the trial (P=.11; Figure 2). No patient required renal replacement therapy.
The mean ± standard deviation/median (IQR) increase of serum creatinine from baseline up to 72 hours after contrast administration was 0.15 ± 0.31/0.10 mg/dL (0-0.2 mg/dL) in the N-acetylcysteine group, 0.17 ± 0.37/0.20 mg/dL (0-0.2 mg/dL) in the ascorbic acid group, and 0.20 ± 0.35/0.20 mg/dL (0-0.5 mg/dL) in the placebo group. This increase was significant (P<.001) in all treatment groups. The eGFR measured 72 hours after angiography also decreased in each treatment group significantly (P<.001 for all three treatment groups). The comparison of serum urea measurements at baseline and 72 hours after contrast exposure failed to demonstrate changes in the N-acetylcysteine group (P=.83) and the ascorbic acid group (P=.68), in contrast to the placebo group which showed a significant increase (P=.04; Figure 3). Figure 4 shows the distributions of the peak serum creatinine increase throughout 72 hours after contrast exposure in each treatment group. The changes between baseline and 72 hours after of serum creatinine, eGFR, and serum urea were not different between the treatment groups: Pserum creatinine = .73, PeGFR
up to 72 hours after contrast administration was 0.15 ± 0.31/0.10 mg/dL (0-0.2 mg/dL) in the N-acetylcysteine group, 0.17 ± 0.37/0.20 mg/dL (0-0.2 mg/dL) in the ascorbic acid group, and 0.20 ± 0.35/0.20 mg/dL (0-0.5 mg/dL) in the placebo group. This increase was significant (P<.001) in all treatment groups. The eGFR measured 72 hours after angiography also decreased in each treatment group significantly (P<.001 for all three treatment groups). The comparison of serum urea measurements at baseline and 72 hours after contrast exposure failed to demonstrate changes in the N-acetylcysteine group (P=.83) and the ascorbic acid group (P=.68), in contrast to the placebo group which showed a significant increase (P=.04; Figure 3). Figure 4 shows the distributions of the peak serum creatinine increase throughout 72 hours after contrast exposure in each treatment group. The changes between baseline and 72 hours after of serum creatinine, eGFR, and serum urea were not different between the treatment groups: Pserum creatinine = .73, PeGFR = .68, Pserum urea = .24. The results of the analysis of the full-analysis set were comparable to the results of the analysis of the per-protocol set.
= .68, Pserum urea = .24. The results of the analysis of the full-analysis set were comparable to the results of the analysis of the per-protocol set.
A logistic regression analysis was performed in order to examine the influence of baseline serum creatinine concentration and amount of contrast dye administered as possible confounders. The dependent variable was the development of CIN and the independent variable of interest was the treatment. Baseline serum creatinine concentration and amount of contrast dye administered failed to predict CIN in the N-acetylcysteine group. When the ascorbic acid group was categorized by median serum creatinine concentration, using the Wald model we could identify a subgroup of patients with a baseline serum creatinine ≤1.4 mg/dL receiving ascorbic acid in which the OR of CIN development (10.6%) is much less than 1 compared to the placebo group (33.7%; P=.0048). The occurrence of CIN in patients with baseline serum creatinine >1.4 mg/dL and receiving ascorbic acid (37.3%) was similar to the placebo group (30.9%; P=.14).
the placebo group (33.7%; P=.0048). The occurrence of CIN in patients with baseline serum creatinine >1.4 mg/dL and receiving ascorbic acid (37.3%) was similar to the placebo group (30.9%; P=.14).
Subgroup Analysis
Effect on patients with diabetes mellitus. The occurrence of diabetes mellitus had no influence on the OR of CIN, when comparing the N-acetylcysteine group with placebo or the ascorbic acid group with placebo, respectively (PN-acetylcysteine vs placebo = .65, Pascorbic acid vs placebo = .62). Among the 236 patients with diabetes mellitus, the incidence of CIN was 35.0% in the placebo group, 28.4% in the N-acetylcysteine group, and 29.8% in the ascorbic acid group.
Adverse events. The safety population consisted of all 520 patients who received at least one dose of a study drug. N-acetylcysteine and ascorbic acid both had good safety and adverse-event profiles. Fifty-five of 520 enrolled patients (10.6%) comprised of 21 patients in the N-acetylcysteine group (10.1%; 95% CI, 6.4%-15.0%), 9 patients in the ascorbic acid group (8.7%; 95% CI, 4.0%-15.8%), and 25 patients in the placebo group (12.0%; 95% CI, 7.9%-17.2%) experienced at least 1 adverse event. All these adverse events in the study were non-serious, self-resolving, and considered to be unrelated to the study drug by the event-adjudication committee. The three treatment groups were associated with a similar number of adverse events (P=.663). Twenty patients terminated the study prematurely, comprised of 9 patients in the N-acetylcysteine group (4.3%; 95% CI, 2.0%-8.1%), 2 patients in the ascorbic acid group (1.9%; 95% CI, 0.2%-6.8%), and 9 patients in the placebo group (4.3%; 95% CI, 2.0%-8.1%) (P=.556).
Discussion
The present study fails to demonstrate that intravenous administration of N-acetylcysteine or ascorbic acid in standard dosages was effective as antioxidative agents to prevent CIN in patients with chronic renal insufficiency undergoing elective cardiac catheterization. These results were consistent among higher-risk patients, such as those with diabetes mellitus and those who received higher amounts of contrast media.
Our findings were in line with recently published, large-scale studies confirming the upcoming evidence based on these high-quality, well-powered trials showing no preventive effect of N-acetylcysteine on the incidence of CIN.36,45 A recent meta-analysis revealed that smaller trials with inadequate methodology tended to overestimate the effect of N-acetylcysteine on the risk of contrast-induced acute kidney injury with important between-trial heterogeneity.45 In the current trial, we sought to ensure adequate methodological quality by using randomization, blinding patients and investigators, analyzing data according to the modified intention-to-treat principle and per-protocol set, and by having 93% of patients with complete follow-up.
The influence of the amount of contrast media administered on the occurrence of renal function deterioration has been a topic of controversy, with recent reports offering opposite conclusions; some show no effect, but most show an increase of the incidence of CIN.36-38,45 In the present study, the amount of contrast agent administered was not an independent predictor of the occurrence of CIN. Compared to other studies with relatively high contrast doses,36,37,45 we used much less contrast media (median value, 110-115 mL), which might help explain the lack of influence of the contrast amount on the occurrence of CIN. There is, however, a general consensus on the use of a small dose of contrast media, and the avoidance of repetitive studies within a small time frame, both recommended to prevent CIN.
The study population, which was comprised of 47% diabetics with impaired renal function, represents a high-risk group for CIN, reflected by the high incidence of CIN in the placebo group (32.1%). In contrast to Kay et al,27post hoc subanalysis of the 236 diabetics in our study indicated that N-acetylcysteine and ascorbic acid were not effective in preventing contrast-induced acute kidney injury, underscoring the lack of effect.
It could be that a substantially higher intravenous dose of N-acetylcysteine than the dose administered in our trial would be effective and might result in a reduced incidence of CIN. Indeed, recently published trials support the hypothesis that high doses of N-acetylcysteine seem more beneficial than standard doses in CIN prevention, both in elective and urgent contrast administration in patients with chronic renal insufficiency.25,26,37 However, in the LIPSIA-N-ACC trial, this dose-dependent effect of high-dose intravenous N-acetylcysteine (total dose, 6000 mg) could not be confirmed.36
Tepel et al29 were the first to report that oral N-acetylcysteine along with hydration is more effective than hydration alone in preventing CIN in patients with chronic kidney disease receiving low-osmolality contrast dye. A difference between our study and that of Tepel was in the protocol for N-acetylcysteine administration. Tepel et al29 gave the drug at 600 mg orally twice daily, the day before and on the day of contrast infusion, while in our study protocol the drug was applied at the same dosage but intravenously. We used intravenous N-acetylcysteine because of a high first-pass effect, resulting in a very low bioavailability of 10%.40 Moreover, we preferred intravenous drug administration to ensure double-blinding due to the sulfurous odor of oral N-acetylcysteine.40 It would seem unlikely that this difference in administration schedule would explain the absence of N-acetylcysteine efficacy in our study.
There is a debate whether the administration of N-acetylcysteine on the day before contrast exposure is useful because orally administered N-acetylcysteine leads to peak serum levels in approximately 1 hour, and the elimination half-life is 2.1 ± 0.8 hours.40 From the standpoint of pharmacokinetics, it is unlikely that administration on the day prior to exposure would be effective. However, since it cannot be ruled out that a metabolite of N-acetylcysteine might have antioxidant or other favorable properties, it is possible that earlier administration could have been beneficial.
The preventive effect of orally high-dose administered ascorbic acid to prevent CIN in patients with chronic renal insufficiency, firstly reported by Spargias et al,43 has not been confirmed by Briguori et al,24 so no conclusive evidence on the effectiveness of ascorbic acid has been provided. In the study by Spargias,43 3 grams of ascorbic acid in chewable tablets or placebo in chewable tablets were supplied at least 2 hours before the start of the procedure, followed by 2 grams the night and the morning after the procedure. It has to be kept in mind that the volume of contrast dye used by Spargias43 was more than twice as high as in our study. We tested a physiological dose of ascorbic acid (500 mg the day before and the day of contrast exposure) administered intravenously due to the low bioavailability after oral administration.46,47 In accordance with Briguori24 using the same high dosage of ascorbic acid as Spargias43 but administered intravenously, we found no benefit of ascorbic acid in physiological dosage administered intravenously. Notably, in a post hoc analysis, ascorbic acid seems to reduce the rate of CIN in patients with mildly impaired renal function (serum creatinine ≤1.4 mg/dL) as compared with saline hydration alone. Although our findings are promising, further data are needed before any conclusions can be made.
Study limitations. The study represents a single-center experience with a limited number of patients. The measurement of serum creatinine within 72 hours of contrast exposure is another potential limitation of the trial because a later increase of serum creatinine level beyond this time interval remained unnoticed. On the other hand, most clinical trials on preventive measures for CIN have demonstrated a peak of serum creatinine level within a time frame of 72 hours after contrast administration, and 90% of CIN cases develop within 72 hours after contrast administration.38 The creatinine clearance was estimated by the MDRD formula, which is widely used in clinical practice; however, it is not a formal measurement of this parameter.
The sample size of this trial was calculated to ascertain a statistically significant difference between the incidence of CIN after placebo versus N-acetylcysteine. The study was underpowered for the comparison of ascorbic acid versus placebo due to practical reasons of this single-center trial making a type II error possible.
Serum creatinine used to assess renal function is not an ideal marker of eGFR estimation because it is eliminated by both glomerular filtration and tubular excretion. Furthermore, serum creatinine concentration is dependent on other factors, such as age, sex and muscle mass. Moreover, creatinine concentration is inaccurate in low serum concentration.48 Finally, some authors also point out that N-acetylcysteine could have a direct influence on serum creatinine concentration independent of eGFR.49 Hence, other markers for eGFR in clinical trials regarding CIN assessment and prevention are required. Cystatin C, for example, is an accurate and promising marker of CIN that describes kidney function more precisely.
Conclusions
In the present study, there is no evidence that standard dosage of N-acetylcysteine or ascorbic acid administered intravenously the day before and the day of contrast dye exposure provides any benefit over placebo with respect to CIN prevention in patients with renal insufficiency undergoing cardiac catheterization. Correct indication for the contrast media administration, periprocedural hydration, the use of a small amount of low-osmolality contrast agent, and the avoidance of repetitive administration of closely spaced contrast dye remain the most important determinants in the prevention of CIN.
References
Berg KJ. Nephrotoxicity related to contrast media. Scand J Urol Nephrol. 2000;34(5):317-322.
Solomon RJ, Natarajan MK, Doucet S, et al. Investigators of the CARE Study. Cardiac angiography in renally impaired patients (CARE) study. A randomized double-blind trial of contrast-induced nephropathy in patients with chronic kidney disease. Circulation. 2007;115(25):3189-3196.
Nikolsky E, Mehran R, Turcot D, et al. Impact of chronic kidney disease on prognosis of patients with diabetes mellitus treated with percutaneous coronary intervention. Am J Cardiol. 2004;94(3):300-305.
Diaz-Sandoval LJ, Kosowsky BD, Losordo DW. Acetylcysteine to prevent angiography-related renal tissue injury (APART trial). Am J Cardiol. 2002;89(3):356-358.
Durham JD, Caputo C, Dokko J, et al. A randomized controlled trial of N-acetylcysteine to prevent contrast nephropathy in cardiac angiography. Kidney Int. 2002;62(6):2202-2207.
Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105(19):2259-2264.
Best PJ, Lennon R, Ting HH, et al. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous interventions. J Am Coll Cardiol. 2002;39(7):1113-1119.
Gruberg L, Mintz GS, Mehran R, et al. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36(5):1542-1548.
Hou SH, Bushinsky DA, Wish JB, et al. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74(2):243-248.
Wang A, Holcslaw T, Bashore TM, et al. Exacerbation of radiocontrast nephrotoxicity by endothelin receptor antagonism. Kidney Int. 2000;57(4):1675-1680.
McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51(15):1419-1428.
Maeder M, Klein M, Fehr T, Rickli H. Contrast nephropathy: review focusing on prevention. J Am Coll Cardiol. 2004;44(9):1763-1771.
Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: a clinical and evidence-based approach. Circulation. 2006;113(14):1799-1806.
McCullough PA, Wolyn R, Rocher LL, et al. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103(5):368-375.
Gruberg L, Mehran R, Dangas G, et al. Acute renal failure requiring dialysis after percutaneous coronary interventions. Catheter Cardiovasc Interv. 2001;52(4):409-416.
Thomsen HS. How to avoid CIN: guidelines from the European Society of Urogenital Radiology. Nephrol Dial Transplant. 2005;20(Suppl 1):i18-i22.
Mehran R, Nikolsky E. Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int. 2006;100(Suppl):S11-S15.
Mueller C, Buerkle G, Buettner HJ, et al. Prevention of contrast-media associated nephropathy: randomized comparison of 2 hydration regimes in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162(3):329-336.
Trivedi HS, Moore H, Nasr S, et al. A randomized prospective trial to assess the role of saline hydration on the development of contast nephropathy. Nephron Clin Pract. 2003;93(1):C29-C34.
McCullough PA, Bertrand ME, Brinker JA, Stacul F. A meta-analysis of the renal safety of isosmolar iodixanol compared with low-osmolar contrast media. J Am Coll Cardiol. 2006;48(4):692-699.
From AM, Al Badarin FJ, McDonald FS, et al. Iodixanol versus low-osmolar contrast media for prevention of contrast induced nephropathy: meta-analysis of randomized, controlled trials. Circ Cardiovasc Interv. 2010;3(4):351-358.
Reed M, Meier P, Tamhane UU, et al. The relative renal safety of iodixanol compared with low-osmolar contrast media: a meta-analysis of randomized controlled trials. JACC Cardiovasc Interv. 2009;2(7):645-654.
Stacul F, Adam A, Becker CR, et al. CIN Consensus Working Panel: strategies to reduce the risk of contrast-induced nephropathy. Am J Cardiol. 2006;98:59K-77K.
Briguori C, Airoldi F, D’Andrea D, et al. Renal insufficiency following contrast media administration trial (REMEDIAL). A randomized comparison of 3 preventive strategies. Circulation. 2007;115(10):1211-1217.
Briguori C, Colombo A, Violante A, et al. Standard vs double dose of N-acetylcysteine to prevent contrast agent associated nephrotoxicity. Eur Heart J. 2004;25(3):206-211.
Baker CS, Wragg A, Kumar S, et al. A rapid protocol for the prevention of contrast-induced renal dysfunction: the RAPPID study. J Am Coll Cardiol. 2003;41(12):2114-2118.
Kay J, Chow WH, Chan TM, et al. Acetylcysteine for prevention of acute deterioration of renal function following elective coronary angiography and intervention. A randomized controlled trial. JAMA. 2003;289(5):553-558.
Bagshaw SM, McAllister FA, Manns BJ, Ghali WA. Acetylcysteine in the prevention of contrast-induced nephropathy. A case study of the pitfalls in the evolution of evidence. Arch Intern Med. 2006;166(2):161-166.
Tepel N, van der Giet N, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343(3):180-184.
Boccalandro F, Amhad M, Smalling RW, Sdringola S. Oral acetylcysteine does not protect renal function from moderate to high dosis of intravenous radiographic contrast. Catheter Cardiovasc Interv. 2003;58(3):336-341.
Shyu KG, Cheng JJ, Kuan P. Acetylcysteine protects against acute renal damage in patients with abnormal renal function undergoing a coronary procedure. J Am Coll Cardiol. 2002;40(8):1383-1388.
Allaqaband T, Tumuluri R, Malik AM, et al. Prospective randomized study of N-acetylcysteine, fenoldopam, and saline for prevention of radiocontrast-induced nephropathy. Catheter Cardiovasc Interv. 2002;57(3):279-283.
Fung JW, Szeto CC, Chan WW, et al. Effect of N-acetylcysteine for prevention of contrast nephropathy in patients with moderate to severe renal insufficiency: a randomized trial. Am J Kidney Dis. 2004;43(5):801-808.
Nallamothu BK, Shojania KG, Saint S, et al. Is acetylcysteine effective in preventing contrast-related nephropathy? A meta-analysis. Am J Med. 2004;117(12):938-947.
Gonzales DA, Norsworthy KJ, Kern SJ, et al. A meta-analysis of N-acetylcysteine in contrast-induced nephrotoxicity: unsupervised clustering to resolve heterogeneity. BMC Med. 2007;5:32.
Thiele H, Hildebrand L, Schirdewahn C, et al. Impact of high-dose N-acetylcystein versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA-N-ACC (prospective, single-blind, placebo-controlled, randomized Leipzig immediate percutaneous coronary intervention acute myocardial infarction N-ACC) trial. J Am Coll Cardiol. 2010;55(20):2201-2209.
Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354(26):2773-2782.
Briguori C, Mangenelli F, Scarpato P, et al. Acetylcysteine and contrast agent-associated nephrotoxicity. J Am Coll Cardiol. 2002;40(2):298-303.
Durham JD, Caputo C, Dokko J, et al. A randomized controlled trial of N-acetylcysteine to prevent contrast nephropathy in cardiac angiography. Kidney Int. 2002;62(6):2202-2207.
Morgan LR, Holdiness MR, Gillen LE. N-acetylcysteine: its bioavailability and interaction with ifosfamide metabolites. Semin Oncol. 1983;10(Suppl 1):S56-S61.
Lloberas N, Torras J, Herrero-Fresneda I, et al. Postischemic renal oxidative stress induces inflammatory response through PAF and oxidized phospholipids: prevention by antioxidant treatment. FASEB J. 2002;16(8):908-910.
Durak I, Ozbek H, Karaayvaz M, Oztürk HS. Cisplatin induces acute renal failure by impairing antioxidant system in guinea pig: effects of antioxidant supplementation on the cisplatin nephrotoxicity. Drug Chem Toxicol. 2002;25(1):1-8.
Spargias K, Alexopoulos E, Kyrzopoulos S, et al. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation. 2004;110(18):2837-2842.
National Kidney Foundation. K/DOQJ. Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1-266.
ACT Investigators: Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography. Main results form the randomized acetylcysteine for contrast-induced nephropathy trial (ACT). Circulation. 2011;124(11):1250-1259.
Blanchard J, Tozer TN, Rowland M. Pharmacokinetic perspectives on megadoses of ascorbic acid. Am J Clin Nutr. 1997;66(5):1165-1171.
Yung S, Mayersohn M, Robinson JB. Ascorbic acid absorption in humans: a comparison among several dosage forms. J Pharm Sci. 1982;71(3):282-285.
Hoste EA, Damen J, Vanholder RC, et al. Assessment of renal function in recently admitted critically ill patients with normal serum creatinine. Nephrol Dial Transplant. 2005;20(4):747-753.
Hoffmann U, Fischereder M, Krüger B, et al. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004;15(2):407-410.











