Use of Embolic Capture Angioplasty for the Treatment of Occluded Superficial Femoral Artery Segments
Abstract: Treatment of peripheral chronic total occlusion (CTO) is one of the most challenging lesion subsets in peripheral revascularization. Advanced wire technology, novel re-entry catheters and imaging techniques help in crossing such lesions. Subintimal dissection using blunt microdissection devices along with true lumen re-entry techniques have added to the success rates of treating peripheral CTOs. After crossing the occlusion, balloon angioplasty and the placement of self-expanding nitinol stents are usually performed. Peripheral embolization is a known complication of peripheral artery interventions, leading to significant lower-extremity ischemia and complications. Such interventions of peripheral CTOs have been shown to have higher rates of distal embolization. Though no dedicated distal embolic protection strategies are currently available for lower-extremity interventions, use of debris capture angioplasty balloon (Proteus™) may be a feasible alternative. We report 3 cases where this device has been used during recanalization of peripheral CTOs.
J INVASIVE CARDIOL 2011;23(11):480-484
Key words: chronic total occlusion, peripheral artery disease, peripheral embolization
__________________________________________
 Estimates of the prevalence of peripheral artery disease (PAD) in the general US adult population vary widely.1,2 These estimates range between 5 and 10 million adults affected. Lower-limb peripheral artery interventions are being performed in growing numbers every year with more novel devices and techniques being developed to improve procedural success and patient safety.3-7 Increasingly, complex patients with multiple comorbidities and severe forms of PAD are being considered for peripheral arterial intervention. Minimizing the risk of potential complications, especially during these high-risk procedures, may improve patient outcomes. Distal embolization is a common complication of peripheral interventions, especially during procedures involving chronic total occlusion (CTO).7,8 It can lead to occlusion of distal vessels, and thereafter cause significant lower-extremity ischemia and tissue loss.8-10 As no dedicated distal embolic protection strategies are available for such interventions, use of the embolic capture angioplasty balloon (Proteus™, Angioslide, Inc.) (Figure 1) may be advantageous during such interventions. The design of the Proteus balloon provides operators with the option of balloon dilation of stenotic lesions along with capture of embolic debris. We report the following 3 cases to demonstrate the use of this balloon in peripheral CTO interventions.
Estimates of the prevalence of peripheral artery disease (PAD) in the general US adult population vary widely.1,2 These estimates range between 5 and 10 million adults affected. Lower-limb peripheral artery interventions are being performed in growing numbers every year with more novel devices and techniques being developed to improve procedural success and patient safety.3-7 Increasingly, complex patients with multiple comorbidities and severe forms of PAD are being considered for peripheral arterial intervention. Minimizing the risk of potential complications, especially during these high-risk procedures, may improve patient outcomes. Distal embolization is a common complication of peripheral interventions, especially during procedures involving chronic total occlusion (CTO).7,8 It can lead to occlusion of distal vessels, and thereafter cause significant lower-extremity ischemia and tissue loss.8-10 As no dedicated distal embolic protection strategies are available for such interventions, use of the embolic capture angioplasty balloon (Proteus™, Angioslide, Inc.) (Figure 1) may be advantageous during such interventions. The design of the Proteus balloon provides operators with the option of balloon dilation of stenotic lesions along with capture of embolic debris. We report the following 3 cases to demonstrate the use of this balloon in peripheral CTO interventions.
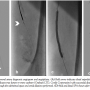 Case 1. A 77-year-old man with diabetes mellitus and severe PAD with long-standing, life-style limiting, left lower-extremity claudication (Rutherford class 3) was referred for diagnostic angiography and intervention. Ankle brachial indices (ABI) on right and left lower extremities were 0.87 and 0.42, respectively. Access to the left superficial femoral artery (SFA) was obtained from a contralateral common femoral arterial approach with a 6 Fr, 45 cm sheath. Diagnostic angiography revealed a long segment of heavily calcified stenosis in the mid SFA with an
Case 1. A 77-year-old man with diabetes mellitus and severe PAD with long-standing, life-style limiting, left lower-extremity claudication (Rutherford class 3) was referred for diagnostic angiography and intervention. Ankle brachial indices (ABI) on right and left lower extremities were 0.87 and 0.42, respectively. Access to the left superficial femoral artery (SFA) was obtained from a contralateral common femoral arterial approach with a 6 Fr, 45 cm sheath. Diagnostic angiography revealed a long segment of heavily calcified stenosis in the mid SFA with an  occluded distal segment. There was single-vessel infra-popliteal run-off. The mid SFA lesion was crossed with a Frontrunner XP (Cordis Corporation) blunt microdissection catheter. The device entered the subintimal space distally. It was then exchanged to an over-the-wire, true lumen re-entry catheter (Outback LTD, Cordis). Successful distal vessel access was secured. After confirming distal access, a 5.0 x 100 mm Proteus balloon was easily delivered to the mid and distal SFA through the subintimal space and serial dilations were performed (Figures 2A-2D). The balloon was then deflated to 2 atm and inward folding and debris capture were performed by retraction of the device handle, following which the balloon was completely deflated and removed. The evacuated balloon was unfolded and debris stained with hematoxylin-eosin on a filter (Figure 3A). Next, the lesion was stented with 2 overlapping distal to proximal self-expanding nitinol stents, and finally the stented segments were postdilated with a 6.0 x 100 mm Ultrathin® (Boston Scientific Corporation) balloon at 10 atm for 10 seconds with an excellent final angiographic result without complications.
occluded distal segment. There was single-vessel infra-popliteal run-off. The mid SFA lesion was crossed with a Frontrunner XP (Cordis Corporation) blunt microdissection catheter. The device entered the subintimal space distally. It was then exchanged to an over-the-wire, true lumen re-entry catheter (Outback LTD, Cordis). Successful distal vessel access was secured. After confirming distal access, a 5.0 x 100 mm Proteus balloon was easily delivered to the mid and distal SFA through the subintimal space and serial dilations were performed (Figures 2A-2D). The balloon was then deflated to 2 atm and inward folding and debris capture were performed by retraction of the device handle, following which the balloon was completely deflated and removed. The evacuated balloon was unfolded and debris stained with hematoxylin-eosin on a filter (Figure 3A). Next, the lesion was stented with 2 overlapping distal to proximal self-expanding nitinol stents, and finally the stented segments were postdilated with a 6.0 x 100 mm Ultrathin® (Boston Scientific Corporation) balloon at 10 atm for 10 seconds with an excellent final angiographic result without complications.
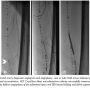 Case 2. A 59-year-old hypertensive man with severe PAD and life-style limiting, bilateral lower-extremity claudication was referred for diagnostic angiography and intervention. ABI on right and left lower extremities were 0.62 and 0.50, respectively. Diagnostic angiography revealed an ostial occlusion of the left SFA with distal reconstitution and triple-vessel infra-popliteal runoff (Figures 4A and 4B). We accessed this lesion with a 6 Fr sheath through a contralateral femoral arterial approach. We crossed the proximal cap of the CTO using a CrossBoss™ (BridgePoint Medical) blunt microdissection catheter, which successfully traversed the CTO and entered the distal true lumen. A 5.0 x 60 mm Proteus balloon was delivered and the lesions dilated. The balloon was then deflated to 2 atm and inward folding and debris capture were performed by retraction of the device handle, following which the balloon was completely deflated and removed (Figures 4C-4E). The evacuated balloon was unfolded and debris stained with hematoxylin-eosin on a filter (Figure 3B). Angiogram performed post-Proteus angioplasty revealed good angiographic result with 40% residual stenosis, which was treated with two overlapping 6.0 x 150 mm self-expanding nitinol stent implantations. The stented segments were postdilated with an excellent final angiographic result.
Case 2. A 59-year-old hypertensive man with severe PAD and life-style limiting, bilateral lower-extremity claudication was referred for diagnostic angiography and intervention. ABI on right and left lower extremities were 0.62 and 0.50, respectively. Diagnostic angiography revealed an ostial occlusion of the left SFA with distal reconstitution and triple-vessel infra-popliteal runoff (Figures 4A and 4B). We accessed this lesion with a 6 Fr sheath through a contralateral femoral arterial approach. We crossed the proximal cap of the CTO using a CrossBoss™ (BridgePoint Medical) blunt microdissection catheter, which successfully traversed the CTO and entered the distal true lumen. A 5.0 x 60 mm Proteus balloon was delivered and the lesions dilated. The balloon was then deflated to 2 atm and inward folding and debris capture were performed by retraction of the device handle, following which the balloon was completely deflated and removed (Figures 4C-4E). The evacuated balloon was unfolded and debris stained with hematoxylin-eosin on a filter (Figure 3B). Angiogram performed post-Proteus angioplasty revealed good angiographic result with 40% residual stenosis, which was treated with two overlapping 6.0 x 150 mm self-expanding nitinol stent implantations. The stented segments were postdilated with an excellent final angiographic result.
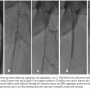 Case 3. A 55-year-old man with hypertension and coronaryartery disease with severe long-standing and life-style limiting bilateral claudication was referred for diagnostic angiography and intervention, and was found to have left SFA occlusion with distal reconstitution in the mid SFA and triple-vessel infrapopliteal runoff (Figure 5A). We again crossed the proximal cap of the SFA with a Treasure wire (Terumo Medical) and a Quick-Cross support catheter (Spectranetics) and used a CrossBoss™ (BridgePoint Medical) to traverse the distal cap and gain access to the true lumen (Figure 5B). Following debulking of the lesion with laser atherectomy (2.0 Turbo Elite®, Spectranetics), the lesion was predilated with a 5.0 x 60 mm Proteus balloon (Figures 5C-5E). Again, the evacuated balloon was unfolded and debris stained with hematoxylin-eosin on a filter (Figure 3C). Following predilation, the residual lesion with a non-flow limiting dissection was treated with a 6.0 x 60 mm nitinol self-expanding stent. Another overlapping 6.0 x 100 mm nitinol stent was placed more proximally. The stented segments were postdilated to achieve an excellent angiographic result with no evidence of distal embolism.
Case 3. A 55-year-old man with hypertension and coronaryartery disease with severe long-standing and life-style limiting bilateral claudication was referred for diagnostic angiography and intervention, and was found to have left SFA occlusion with distal reconstitution in the mid SFA and triple-vessel infrapopliteal runoff (Figure 5A). We again crossed the proximal cap of the SFA with a Treasure wire (Terumo Medical) and a Quick-Cross support catheter (Spectranetics) and used a CrossBoss™ (BridgePoint Medical) to traverse the distal cap and gain access to the true lumen (Figure 5B). Following debulking of the lesion with laser atherectomy (2.0 Turbo Elite®, Spectranetics), the lesion was predilated with a 5.0 x 60 mm Proteus balloon (Figures 5C-5E). Again, the evacuated balloon was unfolded and debris stained with hematoxylin-eosin on a filter (Figure 3C). Following predilation, the residual lesion with a non-flow limiting dissection was treated with a 6.0 x 60 mm nitinol self-expanding stent. Another overlapping 6.0 x 100 mm nitinol stent was placed more proximally. The stented segments were postdilated to achieve an excellent angiographic result with no evidence of distal embolism.
Discussion
Our report indicates that Proteus embolic capture angioplasty balloon catheter could be successfully deployed for the treatment of peripheral arterial CTO lesions in the SFA with embolic debris capture. Our observations support our previously reported finding of distal embolic signals recorded during CTO interventions and the increased risk of peripheral embolic events associated with endovascular treatment of peripheral arterial occlusions.10,11 Distal embolism occurs frequently during interventions, especially procedures involving complex lesions such as thrombotic lesions and long complex occlusions. This feared sequela might either result in abrupt occlusion of large conduit runoff vessels and/or impede microcirculatory flow. Doppler signal recording of distal embolization is registered during 100% of peripheral interventions;12 however, angiographic slowing or cessation of antegrade flow occurs in 3.8-24% of cases.13 Acute or subacute lower-limb ischemia, endovascular treatment of CTOs, and use of atherectomy techniques have also been reported to be independent predictors of distal embolism.14 The SilverHawk Plaque Excision atherectomy device (FoxHollow Technologies) and laser atherectomy have both been linked to distal embolization, with debris collected in distal filter-based embolic protection devices in 20-50% of cases.15 Similarly, angioplasty with provisional stent implantations during infra-inguinal interventions may also be a source of significant embolism. Clinically significant embolism was reported in nearly 30% of angioplasty and/or stent procedures and in 90% of atherectomies reported in the Preventing Lower Extremity Distal Embolization Using Embolic Filter Protection (PRO-TECT) registry.16 In another report, Shammas et al showed that around 2.4% of captured debris require further treatment.11 Given the paucity of data at present to determine when distal embolization needs to be treated, operator judgment is usually the determinant of the course of action taken. Debris resulting in slow or no flow, particularly in high-risk patients with already compromised distal runoffs, usually require further therapy. In a subset analysis of the same registry by Shammas et al, distal embolization was shown to lead to a prolonged procedure time, more contrast use, as well as more fluoroscopy and radiation exposure, factors important to both operators and patients.17
In light of the above, reducing embolic complications during peripheral CTO interventions may improve patient outcomes. In our opinion, the use of embolic capture balloon angioplasty may be best suited for special clinical situations where the risk of distal embolization may be higher: acute or subacute limb ischemia, presence of angiographic filling defects consistent with intraluminal thrombus, lower-extremity arterial CTO, post-atherectomy device applications, and/or in the presence of poor distal vessel runoff or sluggish flow. It is, however, important to add that the rationale for the use of embolic protection strategies along with Proteus angioplasty balloon in these settings is largely based on anecdotal reports and needs to be confirmed by large-scale clinical outcome trials. Moreover, the lack of approved devices for embolic protection during peripheral arterial interventions needs a special mention. This has led to off-label use of distal and proximal embolic protection devices, predominantly indicated for the coronary or saphenous aortocoronary graft locations. The Proteus embolic capture angioplasty balloon may provide the opportunity to deviate away from such practices and facilitate use of an indicated device specifically designed for peripheral arterial interventions and, indirectly, for the first time foster systematic assessment of the risk and efficacy of employing an embolic protection strategy during endovascular treatment of PAD. Data from the MC-LEADER trial18 suggest a strong correlation between lesion complexity and amount of debris evacuated with the Proteus balloon. Overall, debris was removed in over 92% of all peripheral interventions performed. Reports from our group and others18,19 indicate high success rates for this device with respect to lesion dilation and embolic debris capture.
Embolization during a peripheral CTO intervention occurs during crossing and dilation of the occluded segment.10 Despite this recognition, strategies at our disposal to prevent such embolization are currently limited. First, distal embolic strategies cannot be used, as they require first crossing the lesion; second, proximal occlusion catheters (Proxis™, St. Jude Medical) are likely to occlude flow in the proximal segment and/or impede flow to the collateral vessels supplying the distal vasculature, leading to limb ischemia and patient discomfort.
Our report has several important limitations. First and foremost, it includes a small number of patients from a single center. Other important limitations include lack of comparative assessment to other available peripheral arterial angioplasty balloons, other embolic protection strategies in general, and absence of follow-up information. Despite these limitations, we believe that this report demonstrates for the first time the potential use of an angioplasty balloon and embolic capture strategy during high-risk peripheral CTO interventions.
References
- Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317-1324.
- Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110(6):738-743. Epub 2004 Jul 19.
- Baril DT, Marone LK, Kim J. Outcomes of endovascular interventions for TASC II B and C femoropopliteal lesions. J Vasc Surg. 2008;48(3):627-633.
- Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354(18):1879-1888.
- Conrad MF, Cambria RP, Stone DH, et al. Intermediate results of percutaneous endovascular therapy of femoropopliteal occlusive disease: a contemporary series. J Vasc Surg. 2006;44(4):762-769.
- Kedora J, Hohmann S, Garrett W, Munschaur C, Theune B, Gable D. Randomized comparison of percutaneous Viabahn stent grafts versus prosthetic femoral popliteal bypass in the treatment of superficial femoral arterial occlusive disease. J Vasc Surg. 2007;45(1):10-16.
- Mwipatayi BP, Hockings A, Hofmann M, Garbowski M, Sieunarine K. Balloon angioplasty compared with stenting for treatment of femoropopliteal occlusive disease: a meta-analysis. J Vasc Surg. 2008;47(2):461-469.
- Burns BJ, Phillips AJ, Fox A, Boardman P, Phillips-Hughes J. The timing and frequency of complications after peripheral percutaneous transluminal angioplasty and iliac stenting: is a change from inpatient to outpatient therapy feasible? Cardiovasc Intervent Radiol. 2000;23(6):452-456.
- Kudo T, Inoue Y, Nakamura H, Sugano N, Hirokawa M, Iwai T. Characteristics of peripheral microembolization during iliac stenting: Doppler ultrasound monitoring. Eur J Vasc Endovasc Surg. 2005;30(3):311-314.
- Banerjee S, Iqbal A, Sun S, Master R, Brilakis ES. Recording peripheral embolic signals during endovascular treatment of infra-inguinal chronic total occlusion. Cardiovasc Revasc Med. 2011;12(2):134.e7-134e10.
- Shammas NW, Shammas GA, Dippel EJ, Jerin M, Shammas WJ. Predictors of distal embolization in peripheral percutaneous interventions: a report from a large peripheral vascular registry. J Invasive Cardiol. 2009;21(12):628-631.
- Lam RC, Shah S, Faries PL, et al. Incidence and clinical significance of distal embolization during percutaneous interventions involving the superficial femoral artery. J Vasc Surg. 2007;46(6):1155-1159.
- Karnabatidis D, Katsanos K, Kagadis GC, et al. Distal embolism during percutaneous revascularization of infra-aortic arterial occlusive disease: an underestimated phenomenon. J Endovasc Ther. 2006;13(3):269-280.
- Wholey MH, Maynar MA, Wholey MH, et al. Comparison of thrombolytic therapy of lower-extremity acute, subacute, and chronic arterial occlusions. Cathet Cardiovasc Diagn. 1998;44(2):159-169.
- Kaid KA, Gopinathapillai R, Qian F, Salvaji M, Wasty N, Cohen M. Analysis of particulate debris after superficial femoral artery atherectomy. J Invasive Cardiol. 2009;21(1):7-10.
- Shammas NW, Dippel EJ, Coiner D, et al. Preventing lower extremity distal embolization using embolic filter protection: results of the PROTECT registry. J Endovasc Ther. 2008;15(3):270-276.
- Shammas NW, Shammas GA, Dippel E, Jerin M. Intraprocedural outcomes following distal lower extremity embolization in patients undergoing peripheral percutaneous interventions. Vasc Dis Manage. 2009;6:58-61.
- Multi-Center study for Lower Extremity Angioplasty with DEbris Removal (MCLEADER trial) (https://angioslide.com/materials.html).
- Zankar A, Brilakis ES, Banerjee S. Embolic capture angioplasty of lower extremity lesion following distal embolization. Cardiovasc Revasc Med. 2011;12(5):337-340. Epub 2011 May 25.
__________________________________________
From the University of Texas Southwestern Medical Center and VA North Texas Health Care System/University of Texas Southwestern Medical School, Dallas, Texas.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein. Dr. Brilakis is part of the Speaker’s Bureau at St. Jude Medical and Terumo, has received grants from Abbott Vascular and InfraRedx, and salary from Medtronic (spouse). Dr. Banerjee has received grants from Boston Scientific, The Medicine Company, and Cordis Corporation.
Manuscript submitted July 19, 2011, provisional acceptance given August 15, 2011, final version accepted September 7, 2011.
Address for correspondence: Dr. Subhash Banerjee, University of Texas Southwestern Medical Center, 4500 S. Lancaster Road (111a), Dallas, TX 75216. Email: mdcare@aol.com












