Unrestricted Use of Endeavor Resolute Zotarolimus-Eluting Stent in Daily Clinical Practice: A Prospective Registry
Abstract: Background. To evaluate the safety and efficacy of unrestricted Endeavor Resolute zotarolimus-eluting stent (ZES) use. Furthermore, we sought to evaluate clinical outcomes associated with on- and off-label use of Resolute ZES. Methods. The current study was a prospective, single-center registry. The primary endpoint was major adverse cardiac events (MACE), defined as the composite of death, myocardial infarction (MI), and target-vessel revascularization (TVR). Secondary endpoints were death, MI, TVR, and stent thrombosis (ST). Results. A total of 370 patients were prospectively enrolled. Off-label Resolute ZES use was performed in 311 patients (84%). At a mean follow-up of 17.3 ± 6 months, MACE occurred in 31 patients (8.5%), death in 15 (4.1%), MI in 10 (2.7%), and TVR in 19 (5.2%). Definite, probable, and possible ST occurred in 9 patients (2.5%). Off-label Resolute ZES implantation, as compared to on-label use, was not associated with an increased risk of MACE (9.4% vs 3.4%; P=.13), death (4.9% vs 0%; P=.14), MI (3.3% vs 0%; P=.38), and TVR (5.5% vs 3.4%; P=.75). On multivariable analysis, previous revascularization (P=.008), but not off-label Resolute ZES implantation (P=.07), was associated with MACE. Conclusions. In daily clinical practice, Resolute ZES was mostly implanted in patients with off-label indications and associated with a relatively low rate of MACE and TVR.
J INVASIVE CARDIOL 2012;24(6):251-255
Key words: zotarolimus-eluting stent, Endeavor Resolute, percutaneous coronary intervention, off-label, drug-eluting stent
______________________________________________
Drug-eluting stents (DES) represented a breakthrough technology in the invasive treatment of patients with coronary artery disease. Several randomized trials have shown a significant reduction in restenosis, as well as in the need of reintervention, in patients treated with DESs as compared to bare-metal stents.1-3 Nevertheless, concerns have been recently raised about DES safety: several large registries documented that “off-label” DES use, namely DES implantation with indications that have not been tested in first pivotal trials, is associated with a higher incidence of adverse clinical events as compared to “on-label” patients, which have relatively more favorable clinical features and lesion characteristics.4-8 Unfortunately, these findings derived from the evaluation of “first-generation” DESs, such as sirolimus- and paclitaxel-eluting stents. To date, insufficient data are available regarding clinical outcomes associated with the use of a “second-generation” zotarolimus-eluting stent (ZES), namely the Endeavor Resolute ZES (Medtronic Vascular).9 The Resolute ZES employs BioLink polymer technology instead of the phosphorylcholine coating of the first marketed Endeavor ZES, which aims to extend drug elution, thus ensuring better performance even in the treatment of more challenging coronary lesions.10
Therefore, the purpose of this study was to perform a prospective registry evaluating the safety and efficacy of unrestricted Resolute ZES use in a cohort of real-world patients. Furthermore, we sought to evaluate clinical outcomes associated with on- and off-label use of the Resolute ZES.
Methods
Study design and aims. This is a prospective, non-randomized, single-center registry conducted at Federico II University from December 2007 to September 2009. The primary objective of this study was to evaluate the safety and efficacy of the Resolute ZES in a real-world cohort of patients requiring percutaneous coronary intervention (PCI). The secondary objective was to assess whether Resolute ZES implantation in patients with off-label indications is associated with a worse clinical outcome as compared to on-label patients.
Study population and protocol. Inclusion criteria were: (1) patients ≥18 years undergoing routine or emergency PCI; and (2) the presence of at least one documented stenosis >50% in a vessel suitable for PCI with stent implantation. Among the registry population, those patients with clinical and angiographic characteristics that did not encompass the standard-use criteria of pivotal DES studies were included in the off-label group. For the purpose of the current registry, off-label use was defined as Resolute ZES implantation in patients with at least one of the following clinical or lesion characteristics: (1) chronic kidney disease; (2) ejection fraction ≤30%; (3) acute myocardial infarction within previous 72 hours; (4) total occlusion; (5) stent overlapping; (6) lesion length >27 mm; (7) bifurcation coronary lesions; (8) bypass grafts; (9) in-stent restenosis treatment; (10) PCI of stent thrombosis; (11) left main PCI; and (12) thrombus-containing lesion. A femoral approach was used in all cases. Before stent implantation, pharmacologic treatment included the administration of aspirin (as an intravenous loading dose of 500 mg), intravenous heparin (70 IU/kg), and clopidogrel (as a loading dose of 600 mg, or 300 mg for patients who were preloaded). Glycoprotein IIb/IIIa inhibitor use was left to operator’s discretion. In all patients, medical treatment after PCI included beta-blockers, lipid-lowering agents, and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, according to current guidelines. The recommended antiplatelet maintenance regimen was clopidogrel 75 mg/day for at least 12 months and aspirin 100 mg/day indefinitely. Clinical follow-up data were obtained by direct interview at our outpatient clinic or by telephone contact with patients, their relatives, or their referring physicians when necessary.
The study complied with the Declaration of Helsinki regarding investigation in humans and was approved by the Ethics Committee at our clinical institution. All patients provided written informed consent prior to procedure.
Device description. The Endeavor Resolute stent is a next-generation ZES based on a different stent polymer technology and designed to improve clinical outcomes in patients with more complex coronary artery disease.10,11 Like the Endeavor DES system (Medtronic Vascular), the Resolute ZES consists of an antiproliferative drug, zotarolimus, which is a synthetic analog of sirolimus with a similar mechanism of action, and a low-profile, thin-strut, cobalt-chromium alloy stent (Driver stent).12 Conversely, in lieu of the phosphorylcholine-based polymer used in the Endeavor stent, the Resolute ZES employs the BioLink polymer system, consisting of a blend of three different polymers: a hydrophilic and biocompatible C19 polymer, which is crucial in drug elution; a hydrophobic C10 polymer, which improves drug release control; and water soluble polyvinyl pyrrolidone, which allows an early burst of drug release. Although drug concentration remains unchanged (1.6 µg/mm2 of stent surface), at least 85% of zotarolimus is released abluminally within 60 days from stent deployment, while the remaining drug is completely eluted by 180 days.10,11
Study endpoints. The primary, patient-oriented, endpoint of this study was major adverse cardiac events (MACE), defined as the composite of death, myocardial infarction (MI), and target vessel revascularization (TVR). Secondary endpoints were death, MI, TVR, target lesion revascularization (TLR), and stent thrombosis (ST). All deaths were considered cardiac unless an unequivocal non-cardiac cause was established. TVR was defined as any repeat revascularization of the target vessel. TLR was defined as a repeat revascularization within the stent or within 5 mm borders proximal or distal to stent edges. MI and stent thrombosis were defined in compliance with ARC (Academic Research Consortium) criteria.13 According to the timing of event, ST was categorized as early, late, or very late ST, while degree of ST was defined as definite, probable, or possible.13
Statistical analysis. Continuous variables are presented as mean ± standard deviation, and categorical variables as counts and percentages. The normality of distribution of continuous variables was evaluated by the Kolmogorov-Smirnov goodness-of-fit test, and therefore compared with independent sample Student t-test or Mann-Whitney U-test. Categorical variables were compared with chi-square statistic or Fisher’s exact test when appropriate. We derived 95% confidence intervals (CI) for proportions by the Wilson method and the relative risk by the exact method. Exploratory multivariable analysis was done in order to assess the impact of off-label Resolute ZES use on the risk of MACE by a logistic regression analysis. The final model included variables associated at univariate analysis with MACE (P<.15). The results are reported as adjusted hazard ratios (HR) with relative 95% CI. The Hosmer-Lemeshow statistic was performed to evaluate the goodness-of-fit of the logistic regression model. The Kaplan-Meier method was used to calculate the time to clinical endpoints, and the log-rank test was used to compare between-group differences. A P<.05 was considered statistically significant. Statistical analysis was obtained by using SPSS 16.0 statistical package (SPSS Inc).
Results
 A total of 370 patients (mean age, 61.5 ± 11 years; 84.6% males) was prospectively enrolled in this registry. Clinical, angiographic, and procedural characteristics of the study population are listed in Tables 1 and 2. According to study definitions, the Resolute ZES was used for an off-label indication in 311 patients (84%), accounting for 397 lesions (86.3%). Off-label indications to Resolute ZES implantation are reported in Table 3. Acute MI <72 hours represented the most frequent off-label indication, since it was found in 187
A total of 370 patients (mean age, 61.5 ± 11 years; 84.6% males) was prospectively enrolled in this registry. Clinical, angiographic, and procedural characteristics of the study population are listed in Tables 1 and 2. According to study definitions, the Resolute ZES was used for an off-label indication in 311 patients (84%), accounting for 397 lesions (86.3%). Off-label indications to Resolute ZES implantation are reported in Table 3. Acute MI <72 hours represented the most frequent off-label indication, since it was found in 187  patients (50.5%). Patients in the on-label group were more likely to undergo PCI for stable coronary artery disease and had a higher baseline ejection fraction. In contrast, off-label patients were more likely to have a previous PCI history, longer lesions, and more frequently type C lesions treated. As a result, the latter group received more stents, with longer stented segments.
patients (50.5%). Patients in the on-label group were more likely to undergo PCI for stable coronary artery disease and had a higher baseline ejection fraction. In contrast, off-label patients were more likely to have a previous PCI history, longer lesions, and more frequently type C lesions treated. As a result, the latter group received more stents, with longer stented segments.
Clinical follow-up was available in 366 patients (98.9%), with a mean of 17.3 ± 6 months and is reported in Table 4. Follow-up duration was not statistically different between on- and off-label patients (P=.89).
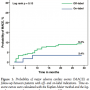
 MACE rate for all patients enrolled in the registry was 8.5% (95% CI, 6%-11.8%). In on-label patients, there was a lower MACE occurrence (3.4%; 95% CI, 0.9%-11.5%) as compared to off-label patients (9.4%; 95% CI, 6.7%-13.2%), but this difference was not statistically significant (P=.13). The relative risk of MACE for the off-label compared with on-label group was 2.97 (95% CI, 0.69-12.8). Kaplan-Meier curves representing the cumulative incidence of MACE at follow-up are shown in Figure 1. TVR rate in the overall
MACE rate for all patients enrolled in the registry was 8.5% (95% CI, 6%-11.8%). In on-label patients, there was a lower MACE occurrence (3.4%; 95% CI, 0.9%-11.5%) as compared to off-label patients (9.4%; 95% CI, 6.7%-13.2%), but this difference was not statistically significant (P=.13). The relative risk of MACE for the off-label compared with on-label group was 2.97 (95% CI, 0.69-12.8). Kaplan-Meier curves representing the cumulative incidence of MACE at follow-up are shown in Figure 1. TVR rate in the overall  cohort was 5.2% (95% CI, 6%-11.8%), with no significant difference (P=.75) between the on-label (3.4%; 95% CI, 0.9%-11.5%) and off-label group (5.5%; 95% CI, 3.5%-8.7%). Death and MI rates in the overall population were 4.1% (95% CI, 2.5%-6.6%) and 2.7% (95% CI, 1.5%-4.9%), respectively (of interest, all cases occurred in off-label patients). Nine patients had ST, with an overall ST rate of 2.5% (95% CI, 1.3%-4.6%). Four cases were early ST and 5 were late ST. Noteworthy, 5 out of 7 definite STs occurred in patients who underwent PCI with overlapping Resolute ZESs. Two late STs were due to premature dual-antiplatelet therapy discontinuation. No very late ST occurred during the study period. Among 185 acute MI patients who completed follow-up, clinical outcomes were: MACE 7% (95% CI, 4.1%-11.6%), death 5.4% (95% CI, 3%-9.7%), MI 2.7% (95% CI, 1.2%-6.2%), TVR 2.7% (95% CI, 1.2%-6.2%), and ST 2.7% (95% CI, 1.2%-6.2%).
cohort was 5.2% (95% CI, 6%-11.8%), with no significant difference (P=.75) between the on-label (3.4%; 95% CI, 0.9%-11.5%) and off-label group (5.5%; 95% CI, 3.5%-8.7%). Death and MI rates in the overall population were 4.1% (95% CI, 2.5%-6.6%) and 2.7% (95% CI, 1.5%-4.9%), respectively (of interest, all cases occurred in off-label patients). Nine patients had ST, with an overall ST rate of 2.5% (95% CI, 1.3%-4.6%). Four cases were early ST and 5 were late ST. Noteworthy, 5 out of 7 definite STs occurred in patients who underwent PCI with overlapping Resolute ZESs. Two late STs were due to premature dual-antiplatelet therapy discontinuation. No very late ST occurred during the study period. Among 185 acute MI patients who completed follow-up, clinical outcomes were: MACE 7% (95% CI, 4.1%-11.6%), death 5.4% (95% CI, 3%-9.7%), MI 2.7% (95% CI, 1.2%-6.2%), TVR 2.7% (95% CI, 1.2%-6.2%), and ST 2.7% (95% CI, 1.2%-6.2%).
 A multivariable model that included insulin-treated diabetes mellitus, three-vessel disease, previous revascularization, and previous MI showed that off-label Resolute ZES implantation did not significantly affect MACE occurrence (HR, 3.96; 95% CI, 0.89-17.6; P=.071). Differently, previous revascularization was associated with MACE (HR, 7.01; 95% CI, 1.41-9.37; P=.008). The Hosmer-Lemeshow statistic was not significant (P=.87), confirming that the model appropriately fit the data.
A multivariable model that included insulin-treated diabetes mellitus, three-vessel disease, previous revascularization, and previous MI showed that off-label Resolute ZES implantation did not significantly affect MACE occurrence (HR, 3.96; 95% CI, 0.89-17.6; P=.071). Differently, previous revascularization was associated with MACE (HR, 7.01; 95% CI, 1.41-9.37; P=.008). The Hosmer-Lemeshow statistic was not significant (P=.87), confirming that the model appropriately fit the data.
Discussion
The main findings of this prospective registry are as follows: (1) in routine clinical practice, most cases of PCI with Resolute ZES implantation were performed in patients with at least one off-label indication; (2) the Resolute ZES represents a safe and effective option for revascularization, with relatively low MACE and TVR rates during the longest available, medium-term follow-up; (3) the rates of MACE, death, TVR, and MI were not significantly different between on- and off-label patients; (4) on multivariable analysis, previous revascularization was associated with MACE; (5) off-label Resolute ZES implantation did not represent an independent risk factor for MACE.
The Resolute ZES, a second-generation ZES, was designed to improve clinical outcomes in patients with more complex coronary artery disease. Currently, the only available clinical data about Resolute ZES performance derive from the Resolute registry9,14 and the Resolute All Comers trial.15
The Resolute registry was a prospective, multicenter, first-in-human study, which enrolled 139 patients with relatively low risk de novo coronary lesions, reporting 12-month MACE rates of 8.7%.14 Although we enrolled patients with a higher risk profile as compared with this registry, our cumulative 8.5% MACE rate was very close to the previously published results, moreover at an extended follow-up time window.
In addition, the 0.7% ST rate found by Meredith et al14 mirrors the absence of ST in the on-label patient cohort of the present registry, albeit fewer overall MACE (3.4%) were observed in this subgroup.
The Resolute All Comers trial15 recruited 2292 patients who were randomized in a 1:1 ratio to receive either Resolute ZES or everolimus-eluting stent. The 12-month results in the Resolute ZES arm (1119 patients) are consistent with our findings; the 8.7% MACE, 4.2% TVR, and 3.9% TLR rates in this randomized trial are very similar to 8.5% MACE, 5.2% TVR, and 3.8% TLR rates observed in our registry. In addition, the 2.3% definite, probable, and possible ST rate is comparable to the 2.5% ST incidence found in the present study. Consistently, at 2-year follow-up, the Resolute ZES confirmed a similar efficacy and safety in comparison with the everolimus-eluting stent, with only 3 cases of very late ST (0.3%).16 A possible reason for these similarities could be the lack of stringent exclusion criteria in the Resolute All Comers trial, which led to the enrollment of patients who are commonly excluded from randomized studies. Indeed, by applying the same definitions, off-label Resolute ZES use occurred in the 67% of patients enrolled in the trial of Serruys et al,15 as well as in the 84% of patients included in this study, with acute MI present in approximately one-half of patients and diabetes in about 34% of cases. Furthermore, most of the definite ST cases occurred in patients with long lesions or treated with multiple overlapping Resolute ZESs due to residual coronary dissection. Consistently, previously published data showed that DES overlapping is an independent predictor of ST17 and is associated with poor clinical outcomes.18
As a matter of fact, DES use in patients with off-label indications still represents a challenging issue, since several studies reported worse clinical outcomes in off-label first-generation DES patients in comparison to on-label patients. Indeed, the Evaluation of Drug Eluting Stents and Ischemic Events (EVENT) registry,7 which evaluated 3323 patients, found that off-label DES use compared to on-label use was associated with a higher MACE incidence at the index admission and at 1 year, with ST also occurring more frequently among off-label patients. Nevertheless, even in our registry, off-label patients had a numerically higher MACE incidence as compared to on-label patients, but this difference was not statistically significant. Furthermore, off-label Resolute ZES use was not associated with MACE on multivariable analysis, suggesting that Resolute ZES implantation in patients presenting with off-label indication might be an acceptable option, without a significant incremental risk of adverse events.
Despite mounting evidence of a very good performance associated with Resolute ZES use, even in very challenging scenarios, whether the Resolute ZES effectively improves clinical outcomes as compared to the Endeavor ZES still remains largely unsolved. The World-Wide Registry with the Endeavor Zotarolimus Eluting Coronary Stent (E-Five) registry is a prospective, multicenter registry, enrolling 8314 patients treated with the Endeavor ZES, which found 12-month death, MI, and ST rates to be 2.4%, 1.6%, and 1.8%, respectively.19 Although the current registry observed similar MACE rates, death, MI, and ST rates are slightly higher than in the E-Five registry. Furthermore, although a so-called Endeavor ZES “extended use” was documented in 74.4% of cases in this multicenter registry, acute MI within 72 hours was present in only 13.9%, while 33.9% of patients had unstable angina.19
Study limitations. This study suffers from the manifest limitations inherent to non-randomized studies. Notwithstanding, it does provide additional data to clinical outcomes associated with Resolute ZES use in a real-world, contemporary setting. Although there was no significant difference in clinical outcomes between on- and off-label patients, and multivariate analysis confirmed that Resolute ZES use in off-label patients was not a risk factor for MACE occurrence, the study remains underpowered to drawn firm conclusions in this regard, and should be taken into account only as hypothesis-generating. Nevertheless, we presented data regarding acute MI as main off-label indication and Resolute ZES confirmed a good safety and efficacy profile in this challenging population. Even if the present registry provides the longest available follow-up of Resolute ZES, a longer-term follow-up is necessary to evaluate late events, which might be not apparent in a mid-term follow-up. Another limitation is the lack of routine angiographic follow-up, which precludes any conclusion about the anti-restenotic effect of Resolute ZES implantation in complex lesions. Nevertheless, we focused on clinically-driven revascularizations, since extensive angiographic follow-up is known to increase the rate of repeat PCI.20
Conclusions
In routine clinical practice, Resolute ZES was mainly implanted in patients with off-label indications and was associated with a relatively low rate of MACE and TVR. However, longer-term follow-up data are required to definitively assess the safety and efficacy of this DES platform.
References
- Kirtane AJ, Gupta A, Iyengar S, et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119(25):3198-3206.
- Piscione F, Piccolo R, Cassese S, et al. Effect of drug-eluting stents in patients with acute ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention: a meta-analysis of randomised trials and an adjusted indirect comparison. EuroIntervention. 2010;5(7):853-860.
- Piscione F, Piccolo R, Cassese S, Galasso G, Chiariello M. Clinical impact of sirolimus-eluting stent in ST-segment elevation myocardial infarction: a meta-analysis of randomized clinical trials. Catheter Cardiovasc Interv. 2009;74(2):323-332.
- Beohar N, Davidson CJ, Kip KE, et al. Outcomes and complications associated with off-label and untested use of drug-eluting stents. JAMA. 2007;297(18):1992-2000.
- Marroquin OC, Selzer F, Mulukutla SR, et al. A comparison of bare-metal and drug-eluting stents for off-label indications. N Engl J Med. 2008;358(4):342-352.
- Pinto Slottow TL, Waksman R. Overview of the 2006 Food and Drug Administration Circulatory System Devices Panel meeting on drug-eluting stent thrombosis. Catheter Cardiovasc Interv. 2007;69(7):1064-1074.
- Win HK, Caldera AE, Maresh K, et al. Clinical outcomes and stent thrombosis following off-label use of drug-eluting stents. JAMA. 2007;297(18):2001-2009.
- Brodie BR, Stuckey T, Downey W, et al. Outcomes and complications with off-label use of drug-eluting stents: results from the STENT (Strategic Transcatheter Evaluation of New Therapies) group. J Am Coll Cardiol. 2008;1(4):405-414.
- Meredith IT, Worthley S, Whitbourn R, et al. The next-generation Endeavor Resolute stent: 4-month clinical and angiographic results from the Endeavor Resolute first-in-man trial. EuroIntervention. 2007;3(1):50-53.
- Udipi K, Melder RJ, Chen M, et al. The next generation Endeavor Resolute stent: role of the BioLinx Polymer System. EuroIntervention. 2007;3(1):137-139.
- Garg S, Serruys PW. Coronary stents: looking forward. J Am Coll Cardiol. 2010;56(10 Suppl):S43-S78.
- Pinto Slottow TL, Waksman R. Overview of the 2007 Food and Drug Administration Circulatory System Devices Panel meeting on the Endeavor zotarolimus-eluting coronary stent. Circulation. 2008;117(12):1603-1608.
- Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344-2351.
- Meredith IT, Worthley S, Whitbourn R, et al. Clinical and angiographic results with the next-generation resolute stent system: a prospective, multicenter, first-in-human trial. J Am Coll Cardiol. 2009;2(10):977-985.
- Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med. 2010;363(2):136-146.
- Silber S, Windecker S, Vranckx P, Serruys PW. Unrestricted randomised use of two new generation drug-eluting coronary stents: 2-year patient-related versus stent-related outcomes from the RESOLUTE All Comers trial. Lancet. 2011;377(9773):1241-1247.
- Costa JR Jr, Sousa A, Moreira AC, et al. Incidence and predictors of very late (>or=4 years) major cardiac adverse events in the DESIRE (drug-eluting stents in the real world)-late registry. J Am Coll Cardiol. 2010;3(1):12-18.
- Raber L, Juni P, Loffel L, et al. Impact of stent overlap on angiographic and long-term clinical outcome in patients undergoing drug-eluting stent implantation. J Am Coll Cardiol. 2010;55(12):1178-1188.
- Lotan C, Meredith IT, Mauri L, Liu M, Rothman MT. Safety and effectiveness of the Endeavor zotarolimus-eluting stent in real-world clinical practice: 12-month data from the E-Five registry. J Am Coll Cardiol. 2009;2(12):1227-1235.
- Uchida T, Popma J, Stone GW, et al. The clinical impact of routine angiographic follow-up in randomized trials of drug-eluting stents: a critical assessment of “oculostenotic” reintervention in patients with intermediate lesions. J Am Coll Cardiol. 2010;3(4):403-411.
______________________________________________
From the Department of Clinical Medicine, Cardiovascular Sciences and Immunology, Federico II University, Naples, Italy.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted November 21, 2011, provisional acceptance given January 2, 2012, final version accepted February 29, 2012.
Address for correspondence: Professor Federico Piscione, MD, Department of Clinical Medicine, Cardiovascular Sciences and Immunology, Federico II University, Via S. Pansini, 5. 80131. Naples, Italy. Email: piscione@unina.it












