Transthoracic Echocardiography as a Measuring and Guiding Tool for Transcatheter Device Closure of Secundum Atrial Septal Defect in Young Children
Abstract: Objective. To analyze the effectiveness of transthoracic echocardiography (TTE) for device closure of secundum atrial septal defect in children ≤5 years old. Study Design. Quasi-experimental study. Study Location and Duration. The study was conducted at Armed Forces Institute of Cardiology and National Institute of Heart Diseases from December 1, 2010 to December 31, 2012. Patients and Methods. During the study period, a total of 48 children ≤5 years old underwent device closure of secundum atrial septal defect. The indications for closure were: elective closure in 31; parental anxiety in 10; frequent respiratory infection in 4; severe pulmonary stenosis in 2; and severe mitral stenosis in 1 patient. The procedure was carried out under general or local anesthesia with TTE and fluoroscopic guidance. TTE was the primary tool used for measurement of defect and estimation of occluder size as well as guiding equipment during device deployment in all patients. Results. A total of 47/48 patients (97.9%) had successful closure of secundum atrial septal defect. The mean age was 4.1 ± .68 years (range, 2.5-5 years) and 28/48 patients (58.4%) were female. The defect size and occluders used were between 5-20 mm (mean, 12 ± 3.5 mm) and 8-22 mm (mean, 15 ± 3.9 mm), respectively. Three patients had simultaneous procedures comprising pulmonary balloon valvuloplasty in 2 patients and percutaneous transmitral commissurotomy in 1 patient. The device embolization occurred in 1 patient; the device was retrieved percutaneously and the patient was referred for surgical closure. The minor complications were residual leak (n = 1), transient bradycardia (n = 4), and first-degree heart block (n = 1). The median procedure time was 30 min (range, 15-100 min) and median fluoroscopic time was 6 min (range, 1.50-45 min). There were no emergency surgical explorations, cardiac perforations, vascular injuries, or deaths during this period. Conclusion. TTE can be used as a primary tool for the measurement of atrial septal defect and guidance during device deployment in young children by skilled and professional hands, yet more experience is awaited.
J INVASIVE CARDIOL 2014;26(6):245-248
Key words: atrial septal defect, device closure, percutaneous transmitral commissurotomy
____________________________
The incidence of congenital heart disease (CHD) in the general population is 8-9 per 1000 live births.1 Atrial septal defect (ASD) accounts for 8%-10% of CHD, while secundum ASD comprises 70% of all ASDs. Most patients remain asymptomatic and are diagnosed after incidental finding of heart murmur.2 However, ASD may lead to a number of complications like arrhythmias, pulmonary hypertension, thromboembolism, heart failure, etc, which necessitates its closure. In asymptomatic patients, closure may be deferred to 2-4 years of age; however, early closure may be necessary in some situations.3 Surgical closure remained the gold standard treatment for ASDs.4 Since the first ASD device closure by King and Mills in 1976,5 the technology of percutaneous closure has rapidly progressed, with increasing success rates and minimal complications.6,7 Device closure of secundum ASD is an effective alternative to surgical repair.8,9
We started device closure of secundum ASD in 2001 at our center and with experience the procedure is now being performed in young children. The objective of this study was to access the effectiveness of transthoracic echocardiography (TTE)for percutaneous closure of secundum ASD in young children.
Methods
Forty-eight consecutive patients from the outpatient department (OPD) who fulfilled the inclusion criteria were included in the study population. A detailed two-dimensional TTE (Philips IE-33) was 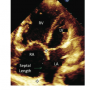 performed using standard protocol for the measurement of defect size, its rims, and total septal length, along with the associated lesions in different views (Figures 1, 2, 3, and 4).
performed using standard protocol for the measurement of defect size, its rims, and total septal length, along with the associated lesions in different views (Figures 1, 2, 3, and 4).
Inclusion criteria. All patients ≤5 years with suitable secundum ASD for device closure were included in the study group. The indications for closure were: elective closure (n = 31); frequent respiratory infections (n = 4); parental anxiety and social reasons (n = 10); pulmonary stenosis (n = 2); and mitral stenosis (n = 1).
Exclusion criteria. We excluded the following: (1) patients >5 years old with secundum ASD; (2) ASD associated with other complex cardiac malformation; (3) ASD with partial anomalous pulmonary venous connection; (4) multifenestrated ASD; and (5) inadequate rims (posterosuperior, posteroinferior, and anteroinferior rim <4 mm).
The occluder size was selected by measuring the defect size in different views by TTE and then 3-5 mm was added to average the defect. In patients where the septal length was challenging for the device (device to septal length ratio was 1:1), we decided to use a sizing balloon with the stop-flow technique under TTE guidance for exact measurement of the defect. In these patients, we only added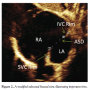 1-2 mm to the defect size for the occluder selection. Eleven out of 48 patients had deficient or absent aortic rim (<3 mm). Two patients had severe pulmonary stenosis, while 1 patient with kyphoscoliosis had severe congenital mitral stenosis (n = 1) and 2 defects were noted in 2 patients.
1-2 mm to the defect size for the occluder selection. Eleven out of 48 patients had deficient or absent aortic rim (<3 mm). Two patients had severe pulmonary stenosis, while 1 patient with kyphoscoliosis had severe congenital mitral stenosis (n = 1) and 2 defects were noted in 2 patients.
The study was approved by the ethical committee of the institute and informed written consent was obtained before the procedure. The procedure was carried out under general or local anesthesia with fluoroscopic and TTE monitoring. The devices were placed in standard fashion and released after confirming the position by TTE in different views, with special focus on rims and atrioventricular valve function (Figure 5). We used four different devices during this study period: Shanghai Shape Memory Alloy (SHSMA; n = 36); Occlutech (n = 5); CardiOFix (n = 5); and Amplatzer septal occluder (AGA Medical; n = 2). All patients were advised to continue oral aspirin (3-5 mg/kg) for 6 months with follow-up at 1, 3, 6, and 12 months.
Hospital stay. All patients stayed for 24 hours in the hospital. TTE was performed at the time of discharge for device position, atrioventricular function, and pericardial effusion.
Statistical analysis. The data were entered in SPSS and the analysis of frequency, percentage, median and mean ± standard deviation of different variables was performed with SPSS version 17. 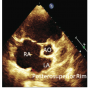
Results
The success rate was 97.9% (47/48) in our study population. The mean age at procedure was 4.1 ± .68 years (range, 2.5-5 years) and mean weight was 13.8 ± 1.9 kg (range, 9.5-19 kg). A total of 28 (58.4%) were female, while 20 (41.6%) were male. The procedure was carried out in 40 patients (83.4%) under general anesthesia, while conscious sedation along with local anesthesia was used in 8 patients. The ASD size was 5-20 mm (mean, 12 ± 3.5 mm) and occluder size was between 8-22 mm (15 ± 3.9 mm). The sizing balloon with stop-flow technique under TTE guidance was used in 3 patients (6.2%) for defect size measurements. The median procedure and fluoroscopy times were 30 min (range, 15-100 min) and 6 min (range, 1.50-45 min), respectively.
Almost a quarter of patients (22.9%, 11/48) with deficient anterosuperior rim had successful closure with right upper pulmonary vein approach and TTE guidance. Two patients (2/48) had severe pulmonary valvular stenosis and underwent pulmonary valvuloplasty along with device closure. The transcatheter pressure gradient was reduced from 60 mm Hg to 16 mm Hg and from 80 mm Hg to 25 mm Hg, respectively. Another patient with kyphoscoliosis and severe congenital mitral stenosis underwent successful percutaneous transmitral commissurotomy (mitral pressure gradient reduced from  21 mm Hg to 10 mm Hg) without any mitral regurgitation. In 2 patients, there were 2 defects that were closed with a single device and there was small (2 mm color jet width) residual leak in 1 patient. There was difficulty in deployment of the device in 4 patients, requiring multiple attempts. In 2 patients, the device could not be deployed even after multiple attempts; balloon sizing with stop-flow technique then revealed a larger defect than the previous one (multiple attempts might have torn the septum). Then, larger-sized devices were deployed with balloon-assisted technique with satisfactory results. In 2 patients, the device appeared to fill the chamber and was replaced by a smaller device. TTE was replaced by transesophageal echocardiography (TEE) in 1 patient for confirmation of device position since the posterosuperior rim was attenuated (~3 mm). The device dislodged immediately after release into the left atrium, which was snared and retrieved successfully percutaneously and referred for surgical closure. The overall minor complications were 12%, including residual leak (n = 1) that settled within 24 hours, transient bradycardia (n = 4), and first-degree heart block (n = 1) during the procedure and recovered without medication. No death occurred during the study period.
21 mm Hg to 10 mm Hg) without any mitral regurgitation. In 2 patients, there were 2 defects that were closed with a single device and there was small (2 mm color jet width) residual leak in 1 patient. There was difficulty in deployment of the device in 4 patients, requiring multiple attempts. In 2 patients, the device could not be deployed even after multiple attempts; balloon sizing with stop-flow technique then revealed a larger defect than the previous one (multiple attempts might have torn the septum). Then, larger-sized devices were deployed with balloon-assisted technique with satisfactory results. In 2 patients, the device appeared to fill the chamber and was replaced by a smaller device. TTE was replaced by transesophageal echocardiography (TEE) in 1 patient for confirmation of device position since the posterosuperior rim was attenuated (~3 mm). The device dislodged immediately after release into the left atrium, which was snared and retrieved successfully percutaneously and referred for surgical closure. The overall minor complications were 12%, including residual leak (n = 1) that settled within 24 hours, transient bradycardia (n = 4), and first-degree heart block (n = 1) during the procedure and recovered without medication. No death occurred during the study period.
Discussion
TTE remained effective as we used it as a primary imaging tool for the measurement of defect size and guiding equipment during device deployment except in 1 case. Wang et al used TTE for guidance with device deployment in 160/165 children <5 years, while they measured the defect with TEE where necessary with a success rate of 98.8%.10 Device closure of secundum ASD remained successful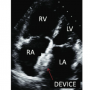 and safe, with few complications in our study population. Although the studies of device closure in young children are lacking, it is still an accepted mode of treatment where necessary.11-13 Diab et al had experience of device closure in 15 infants under TEE and intracardiac echocardiographic guidance and concluded that it is an effective and safe alternative to surgery in infants.14
and safe, with few complications in our study population. Although the studies of device closure in young children are lacking, it is still an accepted mode of treatment where necessary.11-13 Diab et al had experience of device closure in 15 infants under TEE and intracardiac echocardiographic guidance and concluded that it is an effective and safe alternative to surgery in infants.14
The assessment of defect size and guidance for device deployment are key events during the procedure. A number of different imaging tools including transthoracic, transesophageal, and intracardiac echocardiography are available for the measurement of defect size and guidance. TEE remained a standard and old practice for measurement of defect size and confirming the deployment of device closure in many centers.15,16 TEE can result in a number of complications, such as esophageal perforation, dental injuries, posterior pharyngeal wall hematoma, and arrhythmias, especially in young children.17,18 Intracardiac echocardiography is an excellent imaging tool, but currently we are not using it due to non-availability and high cost.19 TTE guidance is safe, effective, and easy during transcatheter ASD closure.20 A number of factors, including size and rims of the defect, septal length, and associated anomalies, are important considerations for successful ASD closure. We carried out a meticulous analysis of all these aspects by TTE. Petit et al pointed out that ASD size to patient weight ratio >1.2 is associated with failure of percutaneous approach in infants and toddlers;21 we did not attempt these cases in order to avoid failures. The device appeared to be oversized in 2 patients and was replaced by a smaller one to prevent long-term complications of atrial and aortic erosions.22,23
Butera et al reported device closure in 48/553 young patients over a 5-year period and had no major complications during the study.24 We also had no major complications; however, 1 device dislodged, and thus our success rate was 97.9% (47/48). Similarly, Cardenas et al reported ASD closure in 52/278 children weighing ≤15 kg over 4.5 years, with procedural success in 94% and no major complications. However, device embolization occurred in 2 patients; the devices were retrieved successfully with snare.25 The minor complications ranged from 10%-16% and were comparable to our minor complication rate (12%). Huang et al26 concluded that deficient anterosuperior rim was a common finding and device closure was effective in these patients. We also observed deficient anterosuperior rim in 11 patients (22.9%) and all had successful device closure.
The procedure time was more than 50 minutes in 5 patients (100 minutes in 1 patient; 75 minutes in 1 patient; 60 minutes in 2 patients; 55 minutes in 1 patient) due to difficult venous access; as also pointed out by Kim et al, relatively small-sized vessels, larger sheaths, and a stiff delivery system potentially risk damage to cardiac tissue in young patients.27 However, there was no tearing, perforation, avulsion, thrombus formation, cardiac tamponade, or death during our study period.
Conclusion
Appropriate case selection is a key to success in young children, since transcatheter device closure of secundum ASD in this age group is safe and effective in experienced hands. TTE can be used as the sole imaging tool for ASD size estimation and guidance of device deployment in these children, since it is easy and effective, yet more experience is awaited.
References
- van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241-2247.
- Porter CJ, Edwards WD. Atrial septal defects. In: Allen HD, Driscoll DJ, Shaddy RE, Feltes TF, eds. Moss and Adams Heart Disease in Infants, Children, and Adolescents. 7th edition. Philadelphia: Lippincott Williams & Wilkins; 2008:635.
- Lammers A, Hager A, Eicken A, et al. Need for closure of secundum atrial septal defect in infancy. J Thorac Cardiovasc Surg. 2005;129(6):1353-1357.
- Baskett RJ, Tancock E, Ross DB. The gold standard for atrial septal defect closure: current surgical results, with an emphasis on morbidity. Pediatr Cardiol. 2003;24(5):444-447.
- King TD, Thompson SL, Steiner L, et al. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA. 1976;235(23):2506-2509.
- Knepp MD, Rocchini AP, Lloyd TR, Aiyagari RM. Long-term follow up of secundum atrial septal defect closure with the Amplatzer septal occluder. Congenit Heart Dis. 2010;5(1):32-37.
- Butera G, Biondi-Zoccai G, Sangiorgi G, et al. Percutaneous versus surgical closure of secundum atrial septal defects: a systematic review and meta-analysis of currently available clinical evidence. EuroIntervention. 2011;7(3):377-385.
- Bialkowski J, Karwot B, Szkutnik M, Banaszak P, Kusa J, Skalski J. Closure of atrial septal defects in children. Surgery versus Amplatzer device implantation. Tex Heart Inst J. 2004;31(3):220-223.
- Du ZD, Hijazi ZM, Kleinman CS, et al; for the Amplatzer Investigators. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults. J Am Coll Cardiol. 2002;39(11):1836-1844.
- Wang HS, Qian MY, Zhang ZW, et al. Applying interventional treatment for the atrial septal defect in 165 children under five years of age. Zhonghua Er Ke Za Zhi. 2005;43(5):373-376.
- Vogel M, Berger F, Dahnert I, et al. Treatment of atrial septal defects in symptomatic children aged less than 2 years of age using the Amplatzer septal occluder. Cardiol Young. 2001;10(5):534-537.
- Choi JY, Kim NK, Park SJ, et al. Feasibility and safety of transcatheter closure of atrial septal defect in small children weighing 10 kg or less. Catheter Cardiovasc Interv. 2008;1:S10.
- Lim DS, Matherne GP. Percutaneous device closure of atrial septal defect in a premature infant with rapid improvement in pulmonary status. Pediatrics. 2007;119(2):398-400.
- Diab KA, Cao QL, Bacha EA, Hijazi ZM. Device closure of atrial septal defects with the Amplatzer septal occluder: safety and outcome in infants. J Thorac Cardiovasc Surg. 2007;134(4):960-966.
- Tseng HC, Hsiao PN, Lin YH, et al. Transesophageal echocardiographic monitoring for transcatheter closure of atrial septal defect. J Formos Med Assoc. 2000;99(9):684-688.
- Mazic U, Gavora P, Masura J. The role of transesophageal echocardiography in transcatheter closure of secundum atrial septal defects by the Amplatzer septal occluder. Am Heart J. 2001;142(3):482-488.
- Mathur SK, Singh P. Transesophageal echocardiography related complications. Indian J Anaesth. 2009:53:267-274.
- Tsigrelis C, Weiner L. Methemoglobinemia revisited: an important complication after transesophageal echocardiography. Eur J Echocardiogr. 2006;7(6):470-472.
- Alboliras ET, Hijazi ZM. Comparison of costs of intracardiac echocardiography and transesophageal echocardiography in monitoring percutaneous device closure of atrial septal defect in children and adults. Am J Cardiol. 2004;94(5):690-692.
- Erdem A, Sarıtas T, Zeybek C, et al. Transthoracic echocardiographic guidance during transcatheter closure of atrial septal defects in children and adults. Int J Cardiovasc Imaging. 2013;29(1):53-61.
- Petit CJ, Justino H, Pignatelli RH, et al. Percutaneous atrial septal defect closure in infants and toddlers. Predictors of success. Pediatr Cardiol. 2013;34(2):220-225.
- Amin Z, Hijazi ZM, Bass JL, et al. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv. 2004;63(4):496-502.
- Lopez-Fernandez T, Gomez de Diego JJ, Monedero MC, et al. Aortic wall erosion after percutaneous closure of atrial septal defect. J Am Soc Echocardiogr. 2011;24(2):227.
- Butera G, De Rosa G, Chessa M, et al. Transcatheter closure of atrial septal defect in young children: results and follow up. J Am Coll Cardiol. 2003;42(2):241-245.
- Cardenas L, Panzer J, Boshoff D, et al. Transcatheter closure of secundum atrial defect in small children. Catheter Cardiovasc Interv. 2007;69(3):447-452.
- Huang CF, Fang CY, Ko SF, et al. Transcatheter closure of atrial septal defects with superior-anterior rim deficiency using Amplatzer septal occluder. J Formos Med Assoc. 2007;106(12):986-991.
- Kim NK, Park SJ, Choi JY. Transcatheter closure of atrial septal defect: does age matter? Korean Circ J. 2011;41(11):633-638.
________________________________
From the Department of Pediatric Cardiology, Armed Forces Institute of Cardiology & National Institute of Heart Diseases (AFIC/NIHD), The Mall, Saddar Rawalpindi, Pakistan.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted July 5, 2013, provisional acceptance given July 31, 2013, final version accepted October 2, 2013.
Address for correspondence: Dr Nadeem Sadiq, Pediatric Cardiology Department, Armed Forces Institute of Cardiology & National Institute of Heart Diseases (AFIC/NIHD) Rawalpindi, Pakistan. Email: drnadeemsadiq@yahoo.com












