Transjugular Percutaneous Transvenous Mitral Commissurotomy (PTMC) Using Conventional PTMC Equipment in Rheumatic Mitral Stenosis With Interruption of Inferior Vena Cava
Abstract: Background. Isolated interruption of the inferior vena cava (IVC) is a rare anomaly. We report a series of 4 cases of isolated interruption of the IVC that coexisted with rheumatic mitral stenosis. Interrupted IVC precludes the use of the femoral approach to percutaneous transseptal mitral commissurotomy (PTMC). We describe the jugular approach to PTMC in such cases using conventional PTMC equipment. Methods and Results. The mean pre-PTMC mitral valve area was 0.85 cm2. Septal puncture was done through the right internal jugular vein with a pediatric Brokenborough needle (Medtronic) using the levophase of pulmonary artery angiogram and the pigtail as guide. The mitral valve was crossed successfully in all cases and appropriately sized Accura balloons (Vascular Concepts) were used for incremental dilatations. Successful balloon dilatation was achieved in all 4 cases (mean post-PTMC mitral valve area of 1.85 cm2) with no complications. Conclusion. The jugular approach appears to be a safe and effective alternative in cases of rheumatic mitral stenosis with IVC anomalies, thereby preventing an otherwise necessary surgery.
J INVASIVE CARDIOL 2012;24(12):675-678
Key words: rheumatic mitral stenosis, transfemoral septal puncture, anomalous IVC, obstructed IVC
______________________________________
Percutaneous transseptal mitral commissurotomy (PTMC) has been well-established as the treatment of choice for rheumatic mitral stenosis with morphologically suitable valves.1 However, the traditional transfemoral method of septal puncture cannot be performed in cases of anomalous or obstructed inferior vena cava (IVC). Isolated interruption of the IVC, though rare, is well described.2 We report a series of 4 cases of isolated interruption of the IVC with coexistent rheumatic mitral stenosis who underwent successful PTMC through the jugular approach.
Methods
 Four patients with isolated interruption of the IVC and coexistent rheumatic mitral stenosis, who were otherwise planned for mitral valve surgery, were considered for PTMC through the jugular approach. Echocardiography was used before the procedure to assess suitability, valve area, presence of left atrial (LA) appendage clot, and degree of mitral regurgitation. PTMC was considered to be successful when the postprocedure echocardiogram showed a mitral valve area >1.5 cm2 or there was a >50% increase from the initial valve area. The clinical and echocardiographic details of the patients are presented in Table 1.
Four patients with isolated interruption of the IVC and coexistent rheumatic mitral stenosis, who were otherwise planned for mitral valve surgery, were considered for PTMC through the jugular approach. Echocardiography was used before the procedure to assess suitability, valve area, presence of left atrial (LA) appendage clot, and degree of mitral regurgitation. PTMC was considered to be successful when the postprocedure echocardiogram showed a mitral valve area >1.5 cm2 or there was a >50% increase from the initial valve area. The clinical and echocardiographic details of the patients are presented in Table 1.
Procedure. Patients were brought to the catheterization laboratory in a fasting state after prior informed consent and premedication. Echocardiography was used throughout the procedure to assess the location of septal puncture, confirm adequacy of valve dilatation, and to quantify the degree of mitral regurgitation. The procedure was performed from the right internal jugular vein approach through an 8 Fr venous sheath. A 6 Fr pigtail was placed through the right femoral artery in the ascending aorta for continuous pressure monitoring and also for guidance during septal puncture.
LA opacification. Through the 8 Fr venous sheath, an NIH catheter (Cordis Corporation) was guided into the pulmonary artery. A pulmonary artery angiogram was done in the left anterior oblique (LAO) projection to delineate the interatrial septum (IAS) in the levophase.
 Septal puncture. A pediatric 6 Fr Mullins sheath (Medtronic) was advanced over a 0.032˝ wire, and through it a 21 G pediatric Brokenborough needle (Medtronic) with a manually modified acute angled tip was advanced (Figure 1). During puncture, the direction indicator of the needle must point in the 7-8 o’clock position, as opposed to the conventional 5 o’clock position. The IAS was punctured in the LAO view using the pigtail as guide. The IAS puncture was done below the lower extent of the pigtail midway between the pigtail and the spine (see Video 1 at invasivecardiology.com). The entry into the left atrium was confirmed and left atrial pressure was measured. Following septal puncture, 3000 IU of heparin was given.
Septal puncture. A pediatric 6 Fr Mullins sheath (Medtronic) was advanced over a 0.032˝ wire, and through it a 21 G pediatric Brokenborough needle (Medtronic) with a manually modified acute angled tip was advanced (Figure 1). During puncture, the direction indicator of the needle must point in the 7-8 o’clock position, as opposed to the conventional 5 o’clock position. The IAS was punctured in the LAO view using the pigtail as guide. The IAS puncture was done below the lower extent of the pigtail midway between the pigtail and the spine (see Video 1 at invasivecardiology.com). The entry into the left atrium was confirmed and left atrial pressure was measured. Following septal puncture, 3000 IU of heparin was given.
Mitral valve dilatation. Two techniques were used to cross the stenotic mitral valve:
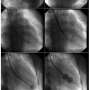 Over–the-wire technique. In case 1, after septal puncture, a 0.025˝ coiled tip wire (Vascular Concepts) was passed through the Mullins sheath in such a manner as to directly enter the left ventricle. The IAS was then dilated using a 14 Fr septal dilator and an appropriately sized Accura balloon (Vascular Concepts) was advanced into the left ventricle over the coil wire (Figure 2).
Over–the-wire technique. In case 1, after septal puncture, a 0.025˝ coiled tip wire (Vascular Concepts) was passed through the Mullins sheath in such a manner as to directly enter the left ventricle. The IAS was then dilated using a 14 Fr septal dilator and an appropriately sized Accura balloon (Vascular Concepts) was advanced into the left ventricle over the coil wire (Figure 2).
Conventional technique. In cases 2, 3, and 4, after septal puncture, the 0.025˝ coiled wire was passed through the Mullins sheath into the left atrium and the septum was dilated using a septal dilator. An appropriately sized Acura balloon was advanced into the left atrium and then maneuvered into the left ventricle over a reshaped stylet (Vascular Concepts) with clockwise rotation (Figure 3).
 Once crossed, balloon dilatation was performed as described for the transfemoral approach. Balloon catheter size and maximum diameter of dilatation was determined using a formula based on body height. Incremental dilatations were performed, starting at a diameter 2-4 mm less than the maximum allowed with constant LA pressure and echocardiographic monitoring. The procedure was stopped when valve area was >1.5 cm2 or >50% increase from the initial valve area was obtained with split commissure/s and laminar flow across the mitral valve.
Once crossed, balloon dilatation was performed as described for the transfemoral approach. Balloon catheter size and maximum diameter of dilatation was determined using a formula based on body height. Incremental dilatations were performed, starting at a diameter 2-4 mm less than the maximum allowed with constant LA pressure and echocardiographic monitoring. The procedure was stopped when valve area was >1.5 cm2 or >50% increase from the initial valve area was obtained with split commissure/s and laminar flow across the mitral valve.
Sheaths were removed immediately and hemostasis was achieved by manual pressure. The patients were discharged after 48 hours.
Results
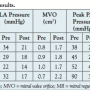 Septal puncture was performed successfully in all 4 cases despite the unfamiliar approach. A high septal puncture (above the fossa ovalis) was achieved in cases 2, 3, and 4, while a low septal puncture was obtained only in case 1. Crossing the mitral valve was easy in cases with a high septal puncture and was successfully performed by clockwise rotation of the balloon. When the septal puncture was low, as in case 1, the over-the-wire technique facilitated easy and direct entry into the left ventricle. Successful balloon dilatation was achieved in all 4 cases with a post-PTMC mitral valve area of >1.5 cm2 obtained in all cases. The mean mitral valve area postprocedure was 1.85 cm2 as compared to the pre-PTMC mean mitral valve area of 0.85 cm2. The increase in valve area was associated with a drop in LA and pulmonary artery pressures. Postprocedure echocardiogram also revealed split commissure/s and significant decrease in mitral valve gradients. (Table 2)
Septal puncture was performed successfully in all 4 cases despite the unfamiliar approach. A high septal puncture (above the fossa ovalis) was achieved in cases 2, 3, and 4, while a low septal puncture was obtained only in case 1. Crossing the mitral valve was easy in cases with a high septal puncture and was successfully performed by clockwise rotation of the balloon. When the septal puncture was low, as in case 1, the over-the-wire technique facilitated easy and direct entry into the left ventricle. Successful balloon dilatation was achieved in all 4 cases with a post-PTMC mitral valve area of >1.5 cm2 obtained in all cases. The mean mitral valve area postprocedure was 1.85 cm2 as compared to the pre-PTMC mean mitral valve area of 0.85 cm2. The increase in valve area was associated with a drop in LA and pulmonary artery pressures. Postprocedure echocardiogram also revealed split commissure/s and significant decrease in mitral valve gradients. (Table 2)
Complications. No major or minor complications were noted in any of our 4 cases. There were no instances of tamponade, significant mitral regurgitation necessitating surgery, or cerebrovascular accidents. All cases were discharged after 48 hours.
Discussion
Isolated interruption of the IVC in the absence of other associated cardiac anomalies is a rare occurrence (prevalence, 0.3%).3,4 At our institute, 1790 PTMC procedures were performed between January 2011 and December 2011, of which only 4 cases had isolated interruption of the IVC. These patients remain asymptomatic and the condition is often accidentally discovered at the time of any intervention or imaging involving the IVC.5
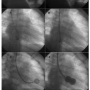
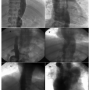 PTMC is an established method of treatment for rheumatic mitral stenosis with morphologically suitable mitral valves.1,6 However, the traditional transfemoral approach to transseptal puncture is not possible in the presence of an interrupted IVC. Our first and fourth cases had interruption of IVC with azygous continuation, while our second case had a left-sided interrupted IVC and hemiazygous continuation. In our third case, the interrupted IVC was drained by both the azygous and the hemiazygous systems (Figure 4).
PTMC is an established method of treatment for rheumatic mitral stenosis with morphologically suitable mitral valves.1,6 However, the traditional transfemoral approach to transseptal puncture is not possible in the presence of an interrupted IVC. Our first and fourth cases had interruption of IVC with azygous continuation, while our second case had a left-sided interrupted IVC and hemiazygous continuation. In our third case, the interrupted IVC was drained by both the azygous and the hemiazygous systems (Figure 4).
The treatment options available when rheumatic mitral stenosis co-exists with interrupted IVC include: retrograde transaortic approach to the mitral valve, percutaneous transhepatic mitral commissurotomy, transjugular approach to septal puncture, or surgical open mitral valvotomy/mitral valve replacement. In the retrograde approach, a special ‘steering’ catheter is used to guide the guidewire and balloon across the aortic and mitral valve.7 Since the entire system has to be delivered via the femoral artery, the risk of injury to the femoral artery increases. Moreover, the passage of balloon through the cordae tendinae can result in chordal rupture and severe mitral regurgitation.7 The transhepatic approach has been described in 1 patient with rheumatic mitral stenosis with an IVC Greenfield filter. However, in view of the risk of liver injury and lack of expertise with transhepatic catheterization, this approach was not considered. The transjugular approach was first described in patients with rheumatic mitral stenosis and anatomical LA distortion as an alternative to the femoral approach.8 Septal puncture was done using the traditional Brokenborough needle in the 45° right anterior oblique (RAO), 2 cm below the upper border of the opacified LA. PTMC was completed using traditional PTMC hardware and Inoue balloon. The authors noted that the adult Brokenborough needle was too long and unwieldy for transjugular PTMC and opined that a shorter needle would make transseptal puncture easier through the jugular approach. In a second paper, the same group reported successful jugular PTMC in 10 patients with anatomical abnormalities, including 1 patient with IVC anomaly. Endry’s pediatric transseptal puncture needle (Cook Cardiology) was used with considerable ease in these patients.9 Compared to the Brokenborough needle (71 cm in length), the Endry’s needle is shorter (30 cm in length) and offers easy maneuverability from the upper limb. In addition, subsequent steps in PTMC were also modified, namely the positioning of a 20 cm, 14 Fr Cook sheath directly into the LA, a balloon floatation catheter to assist LV entry, and the use of the Jomiva balloon for mitral valve dilatation. In the present series, we used a pediatric Brokenborough needle (51 cm in length) for septal puncture and conventional hardware to perform the remaining steps of PTMC. Earlier, Sullebarger et al also reported the successful use of a pediatric Brokenborough needle and conventional hardware to perform transjugular PTMC in 1 patient with rheumatic mitral stenosis complicated by prior liver transplantation and severe IVC distrortion.10 The pediatric Brokenborough offers better maneuverability when compared to the adult needle and is also easily available. The needle tip is manually bent to create a more acute angle which favors septal puncture and prevents the needle from slipping down along the septum. The LA opacification seen in the levophase of the PA angiogram acts as a roadmap. The septum is punctured along the opacified border of the LA in the LAO view midway between the pigtail catheter (placed in the non-coronary sinus) and the vertebral border, a little below the level of the pigtail. In case of a lower septal puncture (as in case 1), we use an ‘over-the-wire’ approach wherein the 0.025˝ wire is directly placed inside the LV instead of the LA. The balloon is then easily guided over the wire into the LV across the mitral valve. This over-the-wire balloon entry into the LV has been used extensively at our institute in femoral PTMCs with difficulty in entering the LV.11 The procedure is safe and the occurrence of LV perforation is extremely rare. Appropriately sized Acura balloons were used for dilatation with successful commissural splitting in all 4 cases. The Accura balloon is similar to the Inoue balloon except that it doesn’t have a vent port. It has been shown to be as effective and safe as the Inoue balloon, while being more economical.12
Although we didn’t have any complications in the 4 cases, the upper-limb approach has some limitations. First, the 51 cm pediatric Brokenborough needle, though shorter than the adult needle, is still slightly difficult to manipulate from the head end. The shorter Endry’s needle could make septal puncture easier, but is subject to availability. Second, since the operator works from the head end of the table, the amount of radiation exposure is greater than the femoral approach. Third, the technique is different from that of femoral PTMC and has a learning curve. For example, (1) the direction indicator of the puncture needle must point in the 7-8 o’clock position instead of the conventional 5 o’clock position; and (2) after septal puncture, the balloon must be rotated in a clockwise manner to facilitate entry into the LV, unlike the conventional anticlockwise approach. The LV entry is further hampered by the lack of fulcrum-like support that the septum provides in the femoral route during the rotation maneuvers. Nevertheless, the upper limb approach is a worthy alternative to the femoral approach in conditions involving an anomolous IVC. In our third case, CMV was advised in 2009 due to lack of experience with the jugular approach at that time. However, when she presented with restenosis two years later, repeat surgery was avoided and successful mitral valve dilatation was achieved percutaneously via the jugular approach. Hence, the jugular approach, in experienced hands, is safe, as effective as the femoral route, and more importantly can prevent a surgical procedure when rheumatic mitral stenosis coexists with interruption of the IVC.
Conclusion
The co-existance of rhematic mitral stenosis and isolated interruption of the IVC with azygous or hemiazygous continuation is a rare occurrence. The condition is often accidentally diagnosed in the cath lab while the patient is undergoing conventional PTMC through the femoral route. In such situations, PTMC can be successfully completed from the right internal jugular vein approach using conventional instruments and a pediatric Brokenborough needle. The procedure is safe, yields similar results to femoral PTMC, and can be used as an alternative to the femoral approach when the IVC is anomalous, thereby preventing an OMV/MVR.
Video is also available at the Journal of Invasive Cardiology multimedia center.
References
- Bonow RO, Carabello BA, Chatterjee K, et al: 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118(15):E523-E661.
- Bartram U, Fischer G, Kramer HH. Congenitally interrupted inferior vena cava without other features of the heterotaxy syndrome: report of five cases and characterization of a rare entity. Pediatr Dev Pathol. 2008;11(4):266-273.
- Sheley RC, Nyberg DA, Kapur R. Azygos continuation of the interrupted inferior vena cava: a clue to prenatal diagnosis of the cardiosplenic syndromes. J Ultrasound Med. 1995;14(5):381-387.
- Anderson RC, Adams Jr P, Burke B. Anomalous inferior vena cava with azygos continuation (infrahepatic interruption of the inferior vena cava). Report of 15 new cases. J Pediatr. 1961;59:370-383.
- Vijayvergiya R, Bhat MN, Kumar RM, Vivekanand SG, Grover A. Azygos continuation of interrupted inferior vena cava in association with sick sinus syndrome. Heart. 2005;91(4):e26.
- Nobuyoshi M, Arita T, Shirai S, et al. Percutaneous balloon mitral valvuloplasty: a review. Circulation. 2009;119(8):e211-e219.
- Stefanidis C, Stratos C, Pitsavos C, et al. Retrograde nontransseptal balloon mitral valvuloplasty: immediate results and long-term follow-up. Circulation. 1992;85(5):1760-1767.
- Joseph G, Baruah DK, Kuruttukulam SV, Chandy ST, Krishnaswami S. Transjugular approach to transseptal balloon mitral valvuloplasty. Cathet Cardiovasc Diagn. 1997;42(2):219-226.
- Joseph G, George OK, Mandalay A, Sathe S .Transjugular approach to balloon mitral valvuloplasty helps overcome impediments caused by anatomical alterations. Cathet Cardiovasc Intervent. 2002;57(3):353-362.
- Sullebarger JT, Coto H, Lopez E, Sayad D, Fontanet HL. Transjugular percutaneous Inoue balloon mitral commissurotomy in a patient with inferior vena cava obstruction after liver transplantation. Cathet Cardiovasc Intervent. 2003;59(2):261-265.
- Manjunath CN, Srinivasa KH, Patil CB, Venkatesh HV, Bhoopal TS, Dhanalakshmi C. Balloon mitral valvuloplasty: our experience with a modified technique of crossing the mitral valve in difficult cases. Cathet Cardiovasc Diagn. 1998;44(1):23-26.
- Manjunath CN, Dorros G, Srinivas KH, et al. The Indian experience of percutaneous transvenous mitral commissurotomy: comparison of the triple lumen (Inoue) and double lumen (Accura) variable sized single balloon with regard to procedural outcome and cost savings. J Intervent Cardiol. 1998;11:107-112.
________________________________________________
From the Department of Cardiology, Sri Jayadeva Institute of Cardiovascular Sciences and Research, Jayanagar 9th block, Bannergatta Road, Bangalore, Karnataka, India.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted March 13, 2012, provisional acceptance given April 14, 2012, final version accepted May 1, 2012
Address for correspondence:: Dr Maneesh K. Rai, Department of Cardiology, Sri Jayadeva Institute of Cardiovascular Sciences and Research, Bangalore, Karnataka, India. Email: drmkrai@gmail.com














