Transcatheter Device Occlusion of a Large Pulmonary Arteriovenous Fistula by Exit Closure: The Road Less Travelled
ABSTRACT: Large pulmonary arteriovenous fistula (PAVF) manifests as cyanosis and predisposes to serious complications of right-to-left shunt, and therefore necessitates early treatment. The emergence of antegrade transcatheter closure of feeding arteries as treatment of choice is limited by inherent risk of either recanalization or reappearance of new feeders and potential risk of systemic embolization. Additional closure of the draining vessel by transcatheter device occlusion should overcome the limitations of conventional antegrade technique. We describe two cases of successful transcatheter closure of a large PAVF by antegrade device closure of feeders as well as transseptal retrograde closure of the exiting channel.
J INVASIVE CARDIOL 2014;26(1):E1-E4
Key words: arteriovenous fistula, transcatheter closure, device
__________________________
PAVFs are abnormal direct communications between pulmonary arteries and pulmonary veins bypassing the capillary circulation with an aneurysmal dilatation. The long-term serious consequences of right-to-left shunt across the large PAVF necessitate early intervention.1,2 Transcatheter coils or device embolization has become the treatment of choice in most cases, replacing surgical intervention.3-6 The conventional transcatheter approach conceptualizes the antegrade closure of the feeding artery by embolization technique and subsequent thrombotic closure of sac. While it is mandatory to close all possible feeders, the antegrade approach always carries the potential risk of recanalization or emergence of new feeders to the fistula.4 Also, the redundant large sac with ongoing thrombus formation poses an additional risk of systemic embolization.7,8 The majority of PAVFs have a single draining channel to the left atrium (LA) regardless of the numbers of arterial feeders.9 Isolated or additional closure of the draining vessel by transcatheter device occlusion should overcome the limitations of conventional antegrade technique.10 We hereby describe two successful cases of sequential and simultaneous transcatheter device closure of arterial feeders as well as draining vessels.
Case Report #1. A 17-year-old male adolescent weighing 38 kg was referred to our institute for evaluation of easy fatigability. On physical examination, he had central cyanosis with 68% SPaO2 and clubbing. There were bilateral symmetrical pulses with normal blood pressure. Chest x-ray revealed homogenous tubular opacity in the right lower zone. Electrocardiogram (ECG) and two-dimensional echocardiographic study were normal. Contrast echocardiography was performed by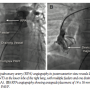 injecting agitated saline in the upper limb peripheral vein. Immediate (within 3 cardiac cycles) appearance of microbubbles in the LA confirmed the provisional diagnosis of PAVF. After informed written consent, right heart catheterization was performed. Selective right pulmonary artery (RPA) angiography with 6 Fr pigtail revealed a large PAVF with more than one feeder, draining into the LA via large vertical tubular vessel parallel to the right border of the LA (Figure 1A). A 6 Fr Judkins right (JR) catheter was advanced over the guidewire into the sac through the largest arterial feeder. The targeted arterial feeder was occluded by deploying a 14 x 16 duct occluder (Block Aid; Shanghai Shape Memory Alloys, Ltd) using 8 Fr Mullins sheath (Cook Corporation). After device deployment, an additional 2 mm feeder was apparent on RPA angiography, in addition to mild foaming through the device (Figure 1B). As oxygen saturation improved from 68% to 92%, the procedure was termed successful and the patient was discharged without procedural complication. At 16-month follow-up exam, the patient had worsening of central cyanosis and SPaO2 was ~78%. Diagnostic RPA angiography in different orthogonal projections revealed 2-3 additional feeders and persistent residual shunt through the stable device (Figure 2A). Probable diagnosis of recanalization of the PAVF was considered. Again, we attempted to close one of the arterial feeders using 0.38" 8-8 embolization coils (Cook
injecting agitated saline in the upper limb peripheral vein. Immediate (within 3 cardiac cycles) appearance of microbubbles in the LA confirmed the provisional diagnosis of PAVF. After informed written consent, right heart catheterization was performed. Selective right pulmonary artery (RPA) angiography with 6 Fr pigtail revealed a large PAVF with more than one feeder, draining into the LA via large vertical tubular vessel parallel to the right border of the LA (Figure 1A). A 6 Fr Judkins right (JR) catheter was advanced over the guidewire into the sac through the largest arterial feeder. The targeted arterial feeder was occluded by deploying a 14 x 16 duct occluder (Block Aid; Shanghai Shape Memory Alloys, Ltd) using 8 Fr Mullins sheath (Cook Corporation). After device deployment, an additional 2 mm feeder was apparent on RPA angiography, in addition to mild foaming through the device (Figure 1B). As oxygen saturation improved from 68% to 92%, the procedure was termed successful and the patient was discharged without procedural complication. At 16-month follow-up exam, the patient had worsening of central cyanosis and SPaO2 was ~78%. Diagnostic RPA angiography in different orthogonal projections revealed 2-3 additional feeders and persistent residual shunt through the stable device (Figure 2A). Probable diagnosis of recanalization of the PAVF was considered. Again, we attempted to close one of the arterial feeders using 0.38" 8-8 embolization coils (Cook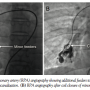 Corporation). However, partial recovery in arterial oxygen saturation from 76% to 84% was consistent with persistent residual flow through the occluded feeders and multiple additional minor feeders to the sac on immediate RPA angiography (Figure 2B). Considering the persistent residual flow and emergence or recruitment of newer channels to the sac, reintervention was planned with the intention to occlude the fistula exit by transseptal approach. After 1 week, draining channel was profiled by selective distal RPA angiography (Figure 3A). After atrial septal puncture, a large, vertical, tubular draining communication was cannulated with a 6 Fr JR. A 10 Fr angulated Mullins sheath was positioned into the fistula over an Amplatzer super-stiff guidewire. An 18 x 16 mm Cardi-O-Fix patent ductus arteriosus (PDA) closure device (Starway Medical Technology, Inc) was deployed midway of the tubular draining vessel (Figure 3B). The minimal opacification of the LA during RPA angiography after device deployment was accompanied by immediate rise in SPaO2 to 96%. At 1-year clinical
Corporation). However, partial recovery in arterial oxygen saturation from 76% to 84% was consistent with persistent residual flow through the occluded feeders and multiple additional minor feeders to the sac on immediate RPA angiography (Figure 2B). Considering the persistent residual flow and emergence or recruitment of newer channels to the sac, reintervention was planned with the intention to occlude the fistula exit by transseptal approach. After 1 week, draining channel was profiled by selective distal RPA angiography (Figure 3A). After atrial septal puncture, a large, vertical, tubular draining communication was cannulated with a 6 Fr JR. A 10 Fr angulated Mullins sheath was positioned into the fistula over an Amplatzer super-stiff guidewire. An 18 x 16 mm Cardi-O-Fix patent ductus arteriosus (PDA) closure device (Starway Medical Technology, Inc) was deployed midway of the tubular draining vessel (Figure 3B). The minimal opacification of the LA during RPA angiography after device deployment was accompanied by immediate rise in SPaO2 to 96%. At 1-year clinical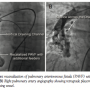 follow-up exam, SPaO2 was maintained at 96%. At 3 years, room air SPaO2 was 94%, and the complete obliteration of PAVF (Figure 3B) was documented on RPA angiography.
follow-up exam, SPaO2 was maintained at 96%. At 3 years, room air SPaO2 was 94%, and the complete obliteration of PAVF (Figure 3B) was documented on RPA angiography.
Case Report #2. A 54-year-old female patient was referred to our institute with complaints of exertional breathlessness for 4 years. At the age of 39 years, she underwent neurosurgery for solitary brain abscess. On physical examination, she had bilateral symmetrical pulses, normal blood pressure and SPaO2 of 84%. ECG, chest x-ray, and two-dimensional echocardiography and Doppler study were unremarkable. As with patient #1, contrast echocardiography raised the possibility of PAVF. Multislice computed tomography pulmonary angiography delineated a large aneurysmal fistulous sac in the anterobasal segment of the right lower lobe (Figure 4A). The large aneurysmal PAVF with a large feeder from the RPA was draining into the LA via a large, horizontal tubular vessel. After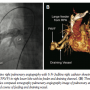 written informed consent, cardiac catheterization was performed with an intention to perform transcatheter closure of the PAVF. Under local anesthesia, right femoral vascular accesses were secured. Unfractionated heparin 100 U/kg and prophylactic antibiotics were administered intravenously. Selective RPA angiography with 6 Fr JR catheter redefined the large PAVF with feeder and draining channels (Figure 4B). A 7 Fr Mullins sheath was positioned at the distal-most segment of the feeder and we transseptally accessed each draining channel. The targeted segment of the arterial feeder and draining channel adjoining the sac were occluded by deploying the 16 mm and 18 mm Amplatzer Vascular Plug II (AGA Corporation), respectively. RPA angiography following simultaneous closure of both the channels by antegrade and retrograde approach revealed complete closure without significant obliteration of nearby branches. The immediate rise in SPaO2 to 98% signaled the successful closure of PAVF by antegrade and retrograde techniques.
written informed consent, cardiac catheterization was performed with an intention to perform transcatheter closure of the PAVF. Under local anesthesia, right femoral vascular accesses were secured. Unfractionated heparin 100 U/kg and prophylactic antibiotics were administered intravenously. Selective RPA angiography with 6 Fr JR catheter redefined the large PAVF with feeder and draining channels (Figure 4B). A 7 Fr Mullins sheath was positioned at the distal-most segment of the feeder and we transseptally accessed each draining channel. The targeted segment of the arterial feeder and draining channel adjoining the sac were occluded by deploying the 16 mm and 18 mm Amplatzer Vascular Plug II (AGA Corporation), respectively. RPA angiography following simultaneous closure of both the channels by antegrade and retrograde approach revealed complete closure without significant obliteration of nearby branches. The immediate rise in SPaO2 to 98% signaled the successful closure of PAVF by antegrade and retrograde techniques.
Discussion. PAVFs can be acquired or congenital. Hereditary hemorrhagic telangiectasia or hereditary generalized angiomatosis are frequent causes of congenital PAVFs, while acquired PAVFs are less frequent.11,12 Indications for PAVF intervention include hypoxemia, neurological symptoms, hemorrhage, progressive enlargement of the lesion, and presence of a 3 mm or larger feeding vessel.4,13-15 Transcatheter embolotherapy has emerged as the treatment of choice for PAVF and has essentially replaced the need for surgery.15 Traditionally, transcatheter embolotherapy of PAVF conceptualizes the antegrade closure of PAVFs by embolization of detachable occlusion balloon, coil, duct occluder, and recently the Amplatzer vascular plug.6,10,19,20 The potential complications of the antegrade approach include air embolism, paradoxical embolization of the device, spontaneous balloon deflation, pleurisy, recanalization, and coil migration.4,15 However, there are three important inherent limitations of the antegrade approach targeting the feeder arteries. First, in patients with complex PAVFs, particularly with multiple feeders, it is mandatory to either close as many feeders as possible to achieve complete closure, or possibly close the very proximal segment of the PA. Second, potential risk of partial or complete recanalization of PAVM after coil closure of targeted vessels always remains. Third, the ongoing thrombotic obliteration of the sac poses the risk of systemic embolization and consequent cerebrovascular stroke.7,8 Almost all PAVFs, including complex PAVFs, drain to the LA by a single large vessel. Isolated closure of the draining channel does achieve closure of the fistula, but there is always theoretical risk of persistent dilatation of the sac. Patient #1 had complex PAVF; with conventional antegrade technique, complete closure could not be achieved despite repeated attempts, while additional device closure of the draining channel during reintervention resulted in technical success and long-lasting clinical success until intermediate-term follow-up. Therefore, in patient #2, an adult patient with history of surgery for brain abscess, both ends of the PAVFs were targeted for simultaneous occlusion using Amplatzer Vascular Plugs delivered by antegrade and transseptal retrograde approaches. Sequential or simultaneous closure of both channels — draining as well as feeding vessels — ensures complete obliteration of the fistulous sac by the enhanced process of sac thrombosis, and at the same time eliminates the future risk of systemic embolization.
symptoms, hemorrhage, progressive enlargement of the lesion, and presence of a 3 mm or larger feeding vessel.4,13-15 Transcatheter embolotherapy has emerged as the treatment of choice for PAVF and has essentially replaced the need for surgery.15 Traditionally, transcatheter embolotherapy of PAVF conceptualizes the antegrade closure of PAVFs by embolization of detachable occlusion balloon, coil, duct occluder, and recently the Amplatzer vascular plug.6,10,19,20 The potential complications of the antegrade approach include air embolism, paradoxical embolization of the device, spontaneous balloon deflation, pleurisy, recanalization, and coil migration.4,15 However, there are three important inherent limitations of the antegrade approach targeting the feeder arteries. First, in patients with complex PAVFs, particularly with multiple feeders, it is mandatory to either close as many feeders as possible to achieve complete closure, or possibly close the very proximal segment of the PA. Second, potential risk of partial or complete recanalization of PAVM after coil closure of targeted vessels always remains. Third, the ongoing thrombotic obliteration of the sac poses the risk of systemic embolization and consequent cerebrovascular stroke.7,8 Almost all PAVFs, including complex PAVFs, drain to the LA by a single large vessel. Isolated closure of the draining channel does achieve closure of the fistula, but there is always theoretical risk of persistent dilatation of the sac. Patient #1 had complex PAVF; with conventional antegrade technique, complete closure could not be achieved despite repeated attempts, while additional device closure of the draining channel during reintervention resulted in technical success and long-lasting clinical success until intermediate-term follow-up. Therefore, in patient #2, an adult patient with history of surgery for brain abscess, both ends of the PAVFs were targeted for simultaneous occlusion using Amplatzer Vascular Plugs delivered by antegrade and transseptal retrograde approaches. Sequential or simultaneous closure of both channels — draining as well as feeding vessels — ensures complete obliteration of the fistulous sac by the enhanced process of sac thrombosis, and at the same time eliminates the future risk of systemic embolization.
Conclusion. Complete thrombotic obliteration and isolation of fistulous sac achieved by transcatheter device closure of both vascular channels (the feeder and draining vessels) is a safe and effective option, eliminating the risk of future embolization and recanalization.
References
- Zeebregts CJ, Nijveld A, Lam J, van Oort AM, Lacquet LK. Surgical treatment of a fistula between the right pulmonary artery and the left atrium: presentation of two cases and review of literature. Eur J Cardiothorac Surg. 1997;11(6):1056-1061.
- Alexi-Meskishvili V, Dähnert I, Ovroutski S, Hetzer R. Right pulmonary artery-to-left atrium communication: a rare cause of systemic cyanosis. Tex Heart Inst J. 2001;28(2):122-124.
- De Giovanni JV.The use of an Amplatzer device to occlude vascular fistulae. J Interv Cardiol. 2001;14(1):45-48.
- Lee DW, White RI Jr, Egglin TK, et al. Embolotherapy of large pulmonary arteriovenous malformations: long-term results. Ann Thorac Surg. 1997;64(4):930-940.
- Takahashi K, Tanimura K, Honda M, et al. Venous sac embolization of pulmonary arteriovenous malformation: preliminary experience using interlocking detachable coils. Cardiovasc Intervent Radiol. 1999;22(3):210-213.
- Apostolopoulou SC, Kelekis NL, Papagiannis J, et al. Transcatheter occlusion of a large pulmonary arteriovenous malformation with use of a Cardioseal device. J Vasc Interv Radiol. 2001:12(6):767-769.
- Felix S, Jeannin S, Goizet C, et al. Stroke following pulmonary arteriovenous fistula embolization in a patient with HHT. Neurology. 2008;71(24):2012-2014.
- Mager JJ, Overtoom TT, Blauw H, Lammers JW, Westermann CJ. Embolotherapy of pulmonary arteriovenous malformations: long-term results in 112 patients. J Vasc Interv Radiol. 2004;15(5):451-456.
- Goodenberger DM. Pulmonary arteriovenous malformation. In: Fishman AP, ed. Fishmans Pulmonary Diseases and Disorders. 4th ed. China: McGraw Hill; 2008:1467-1483.
- Christoph M, Kamal N, Harald B, et al. Embolotherapy in Giant Pulmonary Arteriovenous Malformations. JACC Cardiovasc Interv. 2012;5(9):997-998.
- Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet. 2000;91(1):66-67.
- Plauchu H, de Chadarevian JP, Bideau A, Robert JM. Age-related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am J Med Genet. 1989;32(3):291-297.
- White IR, Lynch-Nylan A, Terry P, et al. PAVFs: techniques and long-term outcome of embolotherapy. Radiology. 1988;169(3):663-669.
- Moussouttas M, Fayad P, Rosenblatt M, et al. Pulmonary arteriovenous malformations: cerebral ischemia and neurologic manifestations. Neurology. 2000;55(7):959-964.
- Dulton TAE, Jackson JE, Hughes JMB, et al. PAVFs results of treatment with coil embolization in 53 patients. AJR Am J Roentogenol. 1995;165(5):1119-1125.
- Pick A, Deschamps C, Stanson AW. Pulmonary arteriovenous fistula: presentation, diagnosis, and treatment. World J Surg. 1999;23(11):1118-1122.
- Faughnan ME, Lui YW, Wirth JA, et al. Diffuse pulmonary arteriovenous malformations: characteristics and prognosis. Chest. 2000;117(1):31-38.
- Swanson KL, Prakash UB, Stanson AW. Pulmonary arteriovenous fistulas: Mayo Clinic experience, 1982–1997. Mayo Clin Proc. 1999;74(7):671-680.
- Ebeid MR, Braden DS, Gaymes CH, et al. Closure of a large pulmonary arteriovenous malformation using multiple Gianturco-Grifka vascular occlusion devices. Catheter Cardiovasc Interv. 2000;49(4):426-429.
- Bialkowski J, Zabal C, Szkutnik M, et al. Percutaneous interventional closure of large pulmonaryarteriovenous fistulas with Amplatzer duct occluder. Am J Cardiol. 2005;96(1):127-129.
From the UN Mehta Institute of Cardiology and Research Centre, Civil Hospital Campus, Asarwa, Ahmedabad, Gujarat, India.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted June 5, 2013 and accepted July 2, 2013.
Address for correspondence: Dr Bhavesh M. Thakkar, Associate Professor and Pediatric Interventional Cardiologist, Department of Pediatric Cardiology, UN Mehta Institute of Cardiology and Research Centre, Ahmedabad, 380016 (Gujarat) India. Email: bthakkarin@yahoo.co.in












