Transcatheter Aortic and Mitral Valve Implantations for Failed Bioprosthetic Heart Valves
Abstract: Background. Restoring degenerated bioprosthetic valves by transcatheter valve implantation may obviate the need for redo surgery in carefully selected patients. We present our initial experience with valve-in-valve (VIV) procedures for failed aortic and mitral bioprosthetic valves. Methods. Data were collected for all patients who underwent VIV procedures at a tertiary medical center (n = 6). Findings were analyzed and compared with those for transcatheter valve implantation in native valves at the same center during the last 3 years (n = 84). Results. Six patients of mean age 78.3 ± 13.8 years (range, 51–87) underwent VIV procedures with the CoreValve (n = 4) or Edwards-SAPIEN device (n = 2). Four (66%) had a failed prosthetic aortic valve, and 2 (33%) had a failed prosthetic mitral valve. Regurgitation accounted for valve failure in 83.3% of the VIV group versus 1.2% of the comparison group (p < 0.001). Procedural success and 30-day survival rates were 100%. Patient functional class improved significantly from 0% class I/II, 50% class III, and 50% class IV before the procedure to 66% class I, 33% class II, and 0% class III/IV after (p < 0.001). Conclusion. This preliminary experience demonstrates that in carefully selected cases, transcathether valve implants can be safely and effectively deployed in stenotic and/or regurgitant degenerated bioprosthetic valves. Further evaluations in larger series are needed.
J INVASIVE CARDIOL 2011;23:377–381
Key words: transcatheter valve implantation, aortic valve replacement, mitral valve replacement
____________________________________
 Transcatheter valve implantation (TVI) has become an accepted alternative treatment for patients with severe symptomatic aortic stenosis who are at a high risk for conventional valve surgery.1–4 Expanding its use to carefully selected patients with degenerated bioprosthetic valves could obviate the need for redo surgery. Factors increasing the risk of reoperation include older patient age, co-morbidity, and technical difficulties caused by the presence of adhesions.5–7 Although bioprosthetic valve failure is common, clinical experience with valve-in-valve (VIV) implantation via catheter is very limited, and it is currently considered “off-label” treatment.8–12 We present our initial experience with transcatheter valve implantation using either the CoreValve System or the Edwards-SAPIEN Transcatheter Heart Valve in failed aortic and mitral bioprosthetic valves using a transfemoral, transaxillary or transapical approach. The patient characteristics and clinical results were compared with TVIs performed in native valves at the same center.
Transcatheter valve implantation (TVI) has become an accepted alternative treatment for patients with severe symptomatic aortic stenosis who are at a high risk for conventional valve surgery.1–4 Expanding its use to carefully selected patients with degenerated bioprosthetic valves could obviate the need for redo surgery. Factors increasing the risk of reoperation include older patient age, co-morbidity, and technical difficulties caused by the presence of adhesions.5–7 Although bioprosthetic valve failure is common, clinical experience with valve-in-valve (VIV) implantation via catheter is very limited, and it is currently considered “off-label” treatment.8–12 We present our initial experience with transcatheter valve implantation using either the CoreValve System or the Edwards-SAPIEN Transcatheter Heart Valve in failed aortic and mitral bioprosthetic valves using a transfemoral, transaxillary or transapical approach. The patient characteristics and clinical results were compared with TVIs performed in native valves at the same center.
Methods
 Study patients. The study group consisted of all patients who underwent a VIV procedure at the Department of Cardiology of a major tertiary medical center since April 2010. Data on patient and procedural characteristics and outcome were collected by file review. The findings were compared with all TVIs performed in the native valve in the same department since the introduction of this technique at our center in November 2008. The technical and procedural success of the TVI procedures and patient quality of life were evaluated according to the criteria of the Valve Academic Research Consortium (VARC).13
Study patients. The study group consisted of all patients who underwent a VIV procedure at the Department of Cardiology of a major tertiary medical center since April 2010. Data on patient and procedural characteristics and outcome were collected by file review. The findings were compared with all TVIs performed in the native valve in the same department since the introduction of this technique at our center in November 2008. The technical and procedural success of the TVI procedures and patient quality of life were evaluated according to the criteria of the Valve Academic Research Consortium (VARC).13
 Statistical analysis. Numerical values are expressed as mean ± standard deviation (SD). Continuous variables were compared between groups with the paired Student t-test (normally distributed variables) or the Wilcoxon test (non-normally distributed). All reported probability values are two-tailed; p < 0.05 was considered statistically significant.
Statistical analysis. Numerical values are expressed as mean ± standard deviation (SD). Continuous variables were compared between groups with the paired Student t-test (normally distributed variables) or the Wilcoxon test (non-normally distributed). All reported probability values are two-tailed; p < 0.05 was considered statistically significant.
Results
 Six patients were treated with the VIV technique since April 2010. Their clinical characteristics are shown in Table 1, and the operative characteristics are shown in Tables 2 and 3. Mean patient age was 78.3 ± 13.8 years (range, 51–87 years). Four patients had a degenerated bioprosthetic aortic valve and 2 had a degenerated bioprosthetic mitral valve. In 83.3% of cases, the main mode of failure was valve regurgitation. The mean Logistic EuroSCORE was 32.4 ± 18.3%.
Six patients were treated with the VIV technique since April 2010. Their clinical characteristics are shown in Table 1, and the operative characteristics are shown in Tables 2 and 3. Mean patient age was 78.3 ± 13.8 years (range, 51–87 years). Four patients had a degenerated bioprosthetic aortic valve and 2 had a degenerated bioprosthetic mitral valve. In 83.3% of cases, the main mode of failure was valve regurgitation. The mean Logistic EuroSCORE was 32.4 ± 18.3%.
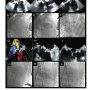 Four procedures were performed with a CoreValve device (2 transfemoral access, 2 transaxillary access) and 2 with an Edwards-SAPIEN device (transapical access). All VIV procedures were technically successful (Figures 1 and 2). None of the patients had vascular problems, stroke, or other major complications. In the aortic procedures, the mean valve gradient decreased from 43 ± 9.3 to 12.3 ± 8.6 mmHg (p < 0.001). Patients were discharged 5.2 ± 1.3 days after the procedure. Thirty-day survival was 100%. There was a significant improvement in patient functional class from before the procedure (0% class I/II, 50% class III, 50% class IV) to after the procedure (66% class I, 33% class II, 0% class III/IV) (p < 0.001).
Four procedures were performed with a CoreValve device (2 transfemoral access, 2 transaxillary access) and 2 with an Edwards-SAPIEN device (transapical access). All VIV procedures were technically successful (Figures 1 and 2). None of the patients had vascular problems, stroke, or other major complications. In the aortic procedures, the mean valve gradient decreased from 43 ± 9.3 to 12.3 ± 8.6 mmHg (p < 0.001). Patients were discharged 5.2 ± 1.3 days after the procedure. Thirty-day survival was 100%. There was a significant improvement in patient functional class from before the procedure (0% class I/II, 50% class III, 50% class IV) to after the procedure (66% class I, 33% class II, 0% class III/IV) (p < 0.001).
 Table 4 compares the findings in study patients with the comparative group of 84 patients who underwent TVI in the native valve in 2008–2011. A CoreValve was implanted in 51 cases (41 transfemoral access, 10 transaxillary access) and an Edwards-SAPIEN valve in 39 (19 transfemoral access, 20 transapical). The number of VIV implantations was too small for statistical analysis of most of the variables. There was a non-significant trend of a higher logistic EuroSCORE in the study group. Valve regurgitation was the mode of failure in 83.3% of the VIV group but only 1.2% of the TVI group (p < 0.001).
Table 4 compares the findings in study patients with the comparative group of 84 patients who underwent TVI in the native valve in 2008–2011. A CoreValve was implanted in 51 cases (41 transfemoral access, 10 transaxillary access) and an Edwards-SAPIEN valve in 39 (19 transfemoral access, 20 transapical). The number of VIV implantations was too small for statistical analysis of most of the variables. There was a non-significant trend of a higher logistic EuroSCORE in the study group. Valve regurgitation was the mode of failure in 83.3% of the VIV group but only 1.2% of the TVI group (p < 0.001).
Discussion
Bioprosthetic valve implantation has become the most common means of surgical valve replacement.14 However, bioprosthetic valve failure, manifested by regurgitation or stenosis, usually occurs within 10–15 years.15,16 TVI is an accepted therapeutic modality for high-surgical-risk patients with severe aortic stenosis in the native valve, but clinical experience with its use in failed bioprosthetic valves is still sparse. Therefore, most patients with a degenerated bioprosthetic valve are either referred for another cardiac surgery, which is uniformly high risk, or treated conservatively, which has a grave prognosis. Balloon valvuloplasty is considered unacceptable because it may lead to disintegration of the prosthetic valve, although sporadic cases have been described.17
Preliminary reports describe the feasibility of VIV procedures as an alternative to repeated valve surgery,9–12 but strong supportive evidence is still lacking. Confirmational studies could lead to a shift in valve selection during surgery, making bioprostheses more attractive than mechanical valves. The lower invasiveness of corrective VIV treatment via catheter relative to conventional surgery would reduce the impact of the early predicted failure of bioprostheses, which is currently their main drawback.
Clinicians should be alerted to several important differences between TVI performed in a native or a bioprosthetic valve. During screening, attention needs to be addressed to the specific features and geometry of the implanted bioprosthetic valve, specifically the external and internal diameter or the valve ring, in order to determine the applicability of TVI in the individual patient. Furthermore, performing balloon inflation inside a bioprosthetic valve before insertion of the transcatheter valve could be dangerous, leading to disintegration of the prosthetic valve with the risk of critical valve regurgitation or stroke. Accordingly, VIV implantation should be performed without prior valvuloplasty. In most cases, the implanted bioprosthesis ring serves as a visible target for placement of the new implant and as a scaffold that grips the valve after implantation. In VIV procedures, the uniform circular sewing ring allows for equal and symmetrical expansion of the skirt of the valve, lessening the risk of para-prosthetic regurgitation.
Our small case series demonstrates that transcatheter CoreValve or Edwards-SAPIEN devices can be safely and effectively deployed in stenotic and regurgitant degenerated surgical aortic and mitral bioprosthetic valves. Further evaluation of large series of TVI procedures in failed bioprosthetic valves is needed.
References
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597-1607.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol. 2004;43(4):698-703.
- Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation. 2007;116(7):755-763.
- Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: Device success and 30-day clinical outcome. J Am Coll Cardiol. 2007;50(1):69-76.
- Akins CW, Buckley MJ, Daggett WM, et al. Risk of reoperative valve replacement for failed mitral and aortic bioprostheses. Ann Thorac Surg. 1998;65(6):1545-1552.
- Jamieson WRE, Burr LH, Miyagishima RT, et al. Re-operation for bioprosthetic aortic structural failure—risk assessment. Eur J Cardiothorac Surg. 2003;24(6):873.
- Eitz T, Fritzsche D, Kleikamp G, et al. Reoperation of the aortic valve in octogenarians. Ann Thorac Surg. 2006;82(4):1385-1391.
- Dainese L, Fusari M, Trabattoni P, Biglioli P. Redo in aortic homograft replacement: Transcatheter aortic valve as a valid alternative to surgical replacement. J Thorac Cardiovasc Surg. 2010;139(6):1656-1657.
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation. 2010;121(16):1848-1857.
- Wenaweser P, Buellesfeld L, Gerckens U, Grube E. Percutaneous aortic valve replacement for severe aortic regurgitation in degenerated bioprosthesis: The first valve in valve procedure using the Corevalve revalving system. Catheter Cardiovasc Interv. 2007;70(5):760-764.
- Khawaja MZ, Haworth P, Ghuran A, et al. Transcatheter aortic valve implantation for stenosed and regurgitant aortic valve bioprostheses CoreValve for failed bioprosthetic aortic valve replacements. J Am Coll Cardiol. 2010;55(2):97-101.
- Gotzmann M, Mugge A, Bojara W. Transcatheter aortic valve implantation for treatment of patients with degenerated aortic bioprostheses—valve-in-valve tech nique. Catheter Cardiovasc Interv. 2010;76(7):1000-1006.
- Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57(3):253-269.
- Brown JM, O’Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137(1):82-90.
- Aupart MR, Mirza A, Meurisse YA, et al. Perimount pericardial bioprosthesis for aortic calcified stenosis: 18-year experience with 1133 patients. J Heart Valve Dis. 2006;15(6):768-775.
- David TE, Ivanov J, Armstrong S, et al. Late results of heart valve replacement with the Hancock II bioprosthesis. J Thorac Cardiovasc Surg. 2001;121(2):268-277.
- Dejam A, Hokinson M, Laham R. Repeated successful balloon valvuloplasty of a bioprosthetic aortic valve in a nonagenerian. Catheter Cardiovasc Interv. 2011;77(4):589-592.
____________________________________
From the 1Department of Cardiology and 2Cardiothoracic Surgery, Rabin Medical Center, Petach Tikva, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel. Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. No authors reported conflicts regarding the content herein. Manuscript submitted May 26, 2011, provisional acceptance given June 13, 2011, final version accepted June 28, 2011. Address for correspondence: Dr. Ran Kornowski, Department of Cardiology, Rabin Medical Center, Petach Tikva 49100, Israel. Email: rkornowski@clalit.org.il












