Successful Transfemoral Antegrade Valve-in-Valve Implantation of a SAPIEN XT Valve into a Degenerated Mitral Valve Prosthesis
Abstract: Transfemoral aortic valve implantation has become an important interventional technique for patients with severe aortic stenosis and high surgical risks. The mitral valve has been a much greater challenge for interventional procedures, and no percutaneous mitral valve implantation system has yet been developed and approved. Here, we report the successful transfemoral, antegrade, valve-in-valve implantation of the SAPIEN XT valve (Edwards Lifesciences) into a degenerated mitral valve bioprosthesis. The patient was an 81-year-old woman with severe regurgitation of her 27 mm Epic mitral valve prosthesis (St. Jude Medical), suffering from dyspnea on mild exertion with a calculated logistic EuroScore of 48% and an Society of Thoracic Surgeons score of 29%. Thus, another surgical mitral valve replacement was refused by the patient and was considered as extremely high risk by the cardiac surgeon. Therefore, a transfemoral valve-in-valve implantation was planned. The femoral vein was used as an access site, followed by a transseptal puncture and placement of a super-stiff wire into the left ventricle. Then, the septum was dilated and a 26 mm SAPIEN XT valve was inserted through the septum and then inserted into the mitral valve prosthesis. Under rapid pacing, a very slow inflation of the balloon was performed, leading to a stable valve-in-valve implantation without any regurgitation. The patient was transferred to a normal ward after 2 days. This procedure is an off-label, technically very challenging, high-risk implantation that could be attempted in very select patients after discussion by a heart team and by experienced implanters.
J INVASIVE CARDIOL 2012;24(4):170-172
Key words: SAPIEN XT, transfemoral aortic valve implantation
___________________________________________
Transfemoral aortic valve implantation (TAVI) has become an important interventional technique for patients with severe aortic stenosis and high surgical risks. The mitral valve has been a much greater challenge for interventional procedures, and no percutaneous mitral valve implantation system has yet been developed and approved. However, transapical valve-in-valve implantations in mitral position have been published for degenerated bioprostheses.1,2 A percutaneous, transfemoral approach for a mitral valve-in-valve implantation has been initially described as unsuccessful,3 and was recently achieved as a first successful procedure by Montorfano et al.4 Himbert et al demonstrated a comparable case into a mitral ring.5 Here, we report about the second successful transfemoral, antegrade valve-in-valve implantation of a SAPIEN XT valve (Edwards Lifesciences) into a degenerated mitral valve bioprosthesis. As a novel approach, this is the first such implantation using the NovaFlex+ delivery catheter (Edwards Lifesciences), a guidewire only in the left ventricle, and an expandable 18 Fr sheath with a valve alignment in the caval vein.
 The patient was an 81-year-old woman with severe regurgitation of her 27 mm Epic mitral valve prosthesis (St. Jude Medical) and suffering from dyspnea on mild exertion (New York Heart Association [NYHA] III). The patient had received an open commissurotomy of the mitral valve for mitral valve stenosis 27 years ago and a mitral valve replacement 7 years ago for mitral valve endocarditis. Concomitant diseases include pulmonary hypertension, renal failure, and severely reduced lung capacity. Her calculated logistic EuroScore was 48% and her Society of Thoracic Surgeons (STS) score was 29%. Thus, another mitral valve replacement was refused by the patient and was considered as extremely high risk by the cardiac surgeon. After careful consideration and discussion with the patient and the cardiac surgeon, a transfemoral valve-in-valve implantation was planned, with informed written consent of the patient. The procedure was performed under general anesthesia. The femoral vein was used as an access site, followed by a transseptal puncture and placement of a super-stiff wire into the left ventricle. Then, the septum was dilated with a 10 mm and 14 mm balloon (Figure 1A) and an 18 Fr expandable sheath was inserted into the femoral vein. A 26 mm Edwards SAPIEN XT prosthesis was reversely mounted on a NovaFlex+ delivery catheter, and the valve alignment was performed in the caval vein (after a previous bench test to evaluate whether inverse valve alignment was feasible). The valve was inserted through the septum (Figure 1B) and then inserted into the mitral valve prosthesis. Under rapid pacing, a very slow inflation of the balloon was performed to ensure optimal placement (Figures 1C and 1D). After removal of the system, the Edwards valve was stable and showed no regurgitation. This was also confirmed after 1 week during a follow-up transesophageal echocardiogram (Figure 2). No residual shunt at the atrial septum was detected. The patient was transferred to a normal ward after 2 days with significantly reduced heart failure symptoms (NYHA I-II).
The patient was an 81-year-old woman with severe regurgitation of her 27 mm Epic mitral valve prosthesis (St. Jude Medical) and suffering from dyspnea on mild exertion (New York Heart Association [NYHA] III). The patient had received an open commissurotomy of the mitral valve for mitral valve stenosis 27 years ago and a mitral valve replacement 7 years ago for mitral valve endocarditis. Concomitant diseases include pulmonary hypertension, renal failure, and severely reduced lung capacity. Her calculated logistic EuroScore was 48% and her Society of Thoracic Surgeons (STS) score was 29%. Thus, another mitral valve replacement was refused by the patient and was considered as extremely high risk by the cardiac surgeon. After careful consideration and discussion with the patient and the cardiac surgeon, a transfemoral valve-in-valve implantation was planned, with informed written consent of the patient. The procedure was performed under general anesthesia. The femoral vein was used as an access site, followed by a transseptal puncture and placement of a super-stiff wire into the left ventricle. Then, the septum was dilated with a 10 mm and 14 mm balloon (Figure 1A) and an 18 Fr expandable sheath was inserted into the femoral vein. A 26 mm Edwards SAPIEN XT prosthesis was reversely mounted on a NovaFlex+ delivery catheter, and the valve alignment was performed in the caval vein (after a previous bench test to evaluate whether inverse valve alignment was feasible). The valve was inserted through the septum (Figure 1B) and then inserted into the mitral valve prosthesis. Under rapid pacing, a very slow inflation of the balloon was performed to ensure optimal placement (Figures 1C and 1D). After removal of the system, the Edwards valve was stable and showed no regurgitation. This was also confirmed after 1 week during a follow-up transesophageal echocardiogram (Figure 2). No residual shunt at the atrial septum was detected. The patient was transferred to a normal ward after 2 days with significantly reduced heart failure symptoms (NYHA I-II).
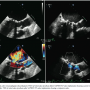 This case represents a novel approach to treat a degenerated mitral valve with a transfemoral, antegrade valve-in-valve implantation. The most challenging aspect of the procedure was the septal and mitral valve passage. This aspect should encourage dilatation of the septum with at least 14-16 mm balloons. Despite having the guidewire only in the left ventricle, we did not experience any difficulties with aligning the SAPIEN XT valve within the mitral valve prosthesis. The newer generation system with a lower catheter profile is clearly beneficial for the optimal alignment. Nevertheless, this procedure remains an off-label, technically very challenging, high-risk implantation that should only be attempted in very select patients after discussion in a heart team and by experienced implanters. Future technical improvements may facilitate this technique.
This case represents a novel approach to treat a degenerated mitral valve with a transfemoral, antegrade valve-in-valve implantation. The most challenging aspect of the procedure was the septal and mitral valve passage. This aspect should encourage dilatation of the septum with at least 14-16 mm balloons. Despite having the guidewire only in the left ventricle, we did not experience any difficulties with aligning the SAPIEN XT valve within the mitral valve prosthesis. The newer generation system with a lower catheter profile is clearly beneficial for the optimal alignment. Nevertheless, this procedure remains an off-label, technically very challenging, high-risk implantation that should only be attempted in very select patients after discussion in a heart team and by experienced implanters. Future technical improvements may facilitate this technique.
In conclusion, transfemoral, antegrade, valve-in-valve implantation of a SAPIEN XT valve into a degenerated mitral bioprosthesis is feasible, using a NovaFlex+ delivery catheter, a guidewire only in the left ventricle and an expandable 18 Fr sheath with a valve alignment on the delivery balloon in the caval vein.
References
- Cheung AW, Gurvitch R, Ye J, et al. Transcatheter transapical mitral valve-in-valve implantations for a failed bioprosthesis: a case series. J Thorac Cardiovasc Surg. 2011;141(3):711-715.
- Seiffert M, Franzen O, Conradi L, et al. Series of transcatheter valve-in-valve implantations in high-risk patients with degenerated bioprostheses in aortic and mitral position. Catheter Cardiovasc Interv. 2010;76(4):608-615.
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation. 2010;121(16):1848-1857.
- Montorfano M, Latib A, Chieffo A, et al. Successful percutaneous antegrade transcatheter valve-in-valve implantation in the mitral position. JACC Cardiovasc Interv. 2011;4(11):1246-1247.
- Himbert D, Brochet E, Radu C, et al. Transseptal implantation of a transcatheter heart valve in a mitral annuloplasty ring to treat mitral repair failure. Circ Cardiovasc Interv. 2011;4(4):396-398.
___________________________________________
From the Department of Cardiology, University of Heidelberg, Heidelberg, Germany.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted December 13, 2011 and accepted January 4, 2012.
Address for correspondence: Raffi Bekeredjian, MD, Department of Cardiology, University of Heidelberg, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany. Email: raffi.bekeredjian@med.uni-heidelberg.de












