ADVERTISEMENT
Subacute Stent Thrombosis Owing to Complete Clopidogrel Resistance Successfully Managed with Prasugrel
ABSTRACT: This report describes a case of an acute anterior myocardial infarction secondary to subacute stent thrombosis of a drug-eluting stent within the proximal segment of the left anterior descending artery (LAD) 5 days after percutaneous transluminal coronary angioplasty and stenting (PCI). The patient was initially managed with conventional dual-antiplatelet therapy (aspirin and clopidogrel) and was subsequently found to have complete absence of adenosine diphosphate (ADP) receptor P2Y12 receptor inhibition. Following additional PCI of the LAD and substitution of clopidogrel for the thienopyridine prasugrel, therapeutic platelet inhibition was achieved without recurrence of stent thrombosis.
J INVASIVE CARDIOL 2011;23:300–304
_________________________________________
The act of performing high-pressure balloon inflations and the advent of peri-procedural dual-antiplatelet therapy [aspirin plus a member of the thienopyridine class of adenosine diphosphate (ADP) receptor P2Y12 inhibitors (ticlopidine, clopidogrel)] has significantly reduced the risk of developing an acute myocardial infarction (MI) due to early or late thrombotic occlusion of a stented coronary artery segment (stent thrombosis; ST). Nevertheless, ST still occurs in 0.6–1.3% of elective cases and in up to 6% of patients with acute coronary syndromes, and thus remains the primary cause of death after percutaneous coronary interventions (PCI) despite the judicious use of antiplatelet therapy.1,2 Although ST can occur acutely in the catheterization lab, most instances of ST are seen in the early (subacute phase occurring from procedure end through 30-day follow-up; 0.6%) and late days following PCI (occurring greater than 30 days; 0.06–0.5%).3,4 Mechanistically, acute and subacute ST are most attributable to lesion-specific and procedural issues, such as residual dissection, stent malapposition/underexpansion, impaired vessel in/out-flow, the use of long stents and stenting of small vessels (< 3.0 mm). In the absence of these features, the primary factor contributing to subacute coronary atherothrombotic events is premature cessation of dual-antiplatelet therapy, namely clopidogrel and/or aspirin,1,4 or more rarely, an intrinsic relative “resistance” to one or both of these agents.5 Although there is no current consensus as to what percentage of patients undergoing PCI inadequately responds to aspirin or clopidogrel (so-called poor- or non-responders), the literature is replete with reports detailing a significantly heightened risk for subacute ST in these patients — presumably a consequence of high residual platelet reactivity.5 Recently, prasugrel, a third-generation thienopyridine, has become available and has shown superior efficacy when compared to clopidogrel, owing to a more rapid onset and greater inhibition of P2Y12 receptor-mediated platelet aggregation.6 Here, we present an unusual case of subacute ST 5 days after coronary artery stenting with laboratory confirmation of complete resistance to clopidogrel therapy.
Case Report
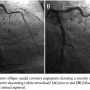 A 45-year-old South Asian male presented initially with unstable angina and underwent PCI of his proximal left anterior descending artery (LAD) (Figure 1A). Briefly, a 0.014˝ Asahi Prowater guidewire (Abbott Vascular, Abbott Park, Illinois) was placed across the LAD lesion into the distal LAD. Using a 20 MHz Eagle Eye Gold intravascular ultrasound (IVUS) imaging system (Volcano Corporation, San Diego, California), the reference vessel diameter was estimated at approximately 3.8 mm proximally and 4.3 mm distally; a notable post-stenotic dilatation can be seen within the diseased segment (Figure 1A). Angioplasty was then performed with a 3.5 mm x 15 mm Voyager balloon (Abbott Vascular) inflated to 15 atm, followed by deployment of a 3.5 mm x 23 mm Xience drug-eluting stent (Abbott Vascular). Finally, the stent was post-dilated with 4.0 and 4.5 mm Quantum Maverick balloons (Boston Scientific, Natick, Massachusetts) proximally and distally, respectively, at high pressures. Figure 1B demonstrates a good angiographic result. Notably, there was no residual dissection visualized angiographically or by IVUS, and both stent apposition and expansion were optimal. The patient had been loaded 3 hours prior to the procedure with 600 mg of clopidogrel and 325 mg of aspirin. Following the procedure, the patient was continued on both clopidogrel (75 mg) and aspirin (325 mg) daily. He was discharged home the following day.
A 45-year-old South Asian male presented initially with unstable angina and underwent PCI of his proximal left anterior descending artery (LAD) (Figure 1A). Briefly, a 0.014˝ Asahi Prowater guidewire (Abbott Vascular, Abbott Park, Illinois) was placed across the LAD lesion into the distal LAD. Using a 20 MHz Eagle Eye Gold intravascular ultrasound (IVUS) imaging system (Volcano Corporation, San Diego, California), the reference vessel diameter was estimated at approximately 3.8 mm proximally and 4.3 mm distally; a notable post-stenotic dilatation can be seen within the diseased segment (Figure 1A). Angioplasty was then performed with a 3.5 mm x 15 mm Voyager balloon (Abbott Vascular) inflated to 15 atm, followed by deployment of a 3.5 mm x 23 mm Xience drug-eluting stent (Abbott Vascular). Finally, the stent was post-dilated with 4.0 and 4.5 mm Quantum Maverick balloons (Boston Scientific, Natick, Massachusetts) proximally and distally, respectively, at high pressures. Figure 1B demonstrates a good angiographic result. Notably, there was no residual dissection visualized angiographically or by IVUS, and both stent apposition and expansion were optimal. The patient had been loaded 3 hours prior to the procedure with 600 mg of clopidogrel and 325 mg of aspirin. Following the procedure, the patient was continued on both clopidogrel (75 mg) and aspirin (325 mg) daily. He was discharged home the following day.
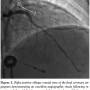
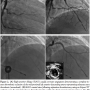 Five days later, the patient developed acute-onset substernal chest pain associated with hypotension and ST-segment elevations in leads V1–V4. Coronary angiography demonstrated complete in-stent thrombotic occlusion of the proximal LAD (Figure 2A). Following intra-aortic balloon pump placement, he underwent emergent aspiration thrombectomy with an Export XT catheter (Medtronic, Minneapolis, Minnesota), followed by balloon angioplasty (3.0 mm Voyager balloon) with partial restoration of TIMI-3 flow (Figure 2B). Further thrombus debulking was achieved with rheolytic therapy following temporary pacemaker placement (AngioJet-Ultra thrombectomy catheter; Medrad, Minneapolis, Minnesota) (Figures 2C–2D). Copious amounts of intracoronary nicardipine and adenosine were given to treat any distal embolization. Further IVUS evaluation revealed an adherent filling defect in the distal stent identified as thrombus (Figure 2D). Therefore, re-stenting was performed in an overlapping fashion across the distal edge of the stent using a 4.0 mm x 12 mm Xience stent deployed at nominal pressure and post-dilated with 4.5 mm and 5.0 mm x 15 mm Quantum Maverick balloons proximally and distally, respectively, with a very good angiographic result (Figure 3) confirmed once again with IVUS.
Five days later, the patient developed acute-onset substernal chest pain associated with hypotension and ST-segment elevations in leads V1–V4. Coronary angiography demonstrated complete in-stent thrombotic occlusion of the proximal LAD (Figure 2A). Following intra-aortic balloon pump placement, he underwent emergent aspiration thrombectomy with an Export XT catheter (Medtronic, Minneapolis, Minnesota), followed by balloon angioplasty (3.0 mm Voyager balloon) with partial restoration of TIMI-3 flow (Figure 2B). Further thrombus debulking was achieved with rheolytic therapy following temporary pacemaker placement (AngioJet-Ultra thrombectomy catheter; Medrad, Minneapolis, Minnesota) (Figures 2C–2D). Copious amounts of intracoronary nicardipine and adenosine were given to treat any distal embolization. Further IVUS evaluation revealed an adherent filling defect in the distal stent identified as thrombus (Figure 2D). Therefore, re-stenting was performed in an overlapping fashion across the distal edge of the stent using a 4.0 mm x 12 mm Xience stent deployed at nominal pressure and post-dilated with 4.5 mm and 5.0 mm x 15 mm Quantum Maverick balloons proximally and distally, respectively, with a very good angiographic result (Figure 3) confirmed once again with IVUS.
 Post-procedural clopidogrel was continued at 75 mg twice daily. Of note, he received no therapies with known thienopyridine drug-drug interactions, namely, proton-pump inhibitors.7,8 Given the patient’s unique and dangerous presentation, unfractionated heparin was continued and platelet function studies were initiated using the Accumetrics VerifyNow P2Y12® rapid analyzer platelet function assay (Accumetrics, Inc., San Diego, California) in search of suspected drug resistance to clopidogrel.
Post-procedural clopidogrel was continued at 75 mg twice daily. Of note, he received no therapies with known thienopyridine drug-drug interactions, namely, proton-pump inhibitors.7,8 Given the patient’s unique and dangerous presentation, unfractionated heparin was continued and platelet function studies were initiated using the Accumetrics VerifyNow P2Y12® rapid analyzer platelet function assay (Accumetrics, Inc., San Diego, California) in search of suspected drug resistance to clopidogrel.
Accumetrics VerifyNow® Platelet Function Testing
Our patient was maintained on clopidogrel (600 mg bolus prior to catheterization and 75 mg once daily, every day thereafter) from the index procedure 5 days prior to his acute presentation and for the next 7 days following his second PCI (where another 600 mg bolus was given, followed by 75 mg twice daily, every day thereafter) in addition to adjunctive intravenous unfractionated heparin and aspirin 325 mg every day. To prevent interference with our platelet function testing, whole blood samples for all laboratory studies including platelet reactivity testing were obtained 7 days following cessation of the glycoprotein IIb/IIIa inhibitor eptifibatide used during his acute presentation and PCI (ST). The inhibitory effect of clopidogrel in our patient was measured using the VerifyNow P2Y12 rapid analyzer, according to the manufacturer’s test protocol, which has been described in detail by others.9 Briefly, the VerifyNow system is a cartridge-based rapid platelet-function assay designed to directly measure the effects of thienopyridines on the P2Y12 platelet receptor. The VerifyNow instrument measures platelet-induced aggregation as an increase in light transmittance and uses a proprietary algorithm to report values in P2Y12 reaction units (PRU). A higher PRU reflects less platelet inhibition or greater ADP-mediated platelet reactivity (high on-treatment reactivity) and a low PRU reflects greater platelet inhibition or less ADP-mediated platelet reactivity (low on-treatment reactivity). The VerifyNow test was then repeated on day 8 (the following day) for confirmation. On day 8, clopidogrel and unfractionated heparin were stopped following administration of a 60 mg loading dose of prasugrel. One hour after prasugrel loading, its platelet inhibitory effect was measured using the VerifyNow P2Y12 rapid analyzer.
 Pharmacologic platelet inhibition in response to thienopyridine treatment is depicted in Table 1. After a total of 12 days of clopidogrel treatment, including 1,200 mg in loading doses and twice-daily dosing after his acute presentation, our patient demonstrated 0% platelet inhibition with the same (yet, numerically higher) relative PRUs in the test result as in the baseline values. Conversely, within 1 hour of prasugrel loading, our patient demonstrated 76% platelet inhibition and an 80% reduction in PRUs. Moreover, as depicted in Table 2, no obvious underlying
Pharmacologic platelet inhibition in response to thienopyridine treatment is depicted in Table 1. After a total of 12 days of clopidogrel treatment, including 1,200 mg in loading doses and twice-daily dosing after his acute presentation, our patient demonstrated 0% platelet inhibition with the same (yet, numerically higher) relative PRUs in the test result as in the baseline values. Conversely, within 1 hour of prasugrel loading, our patient demonstrated 76% platelet inhibition and an 80% reduction in PRUs. Moreover, as depicted in Table 2, no obvious underlying  acquired or genetic thrombophilias appear to be contributing to the development of stent thrombosis in our patient. He remained on prasugrel for the following 4–6 months without incident. These results indicate complete resistance to the antiplatelet effect of clopidogrel, an effect that was rescued with prasugrel treatment in our patient. Optimal diagnostic cut-off measures used here are congruent with previous reports utilizing the VerifyNow system.7,10
acquired or genetic thrombophilias appear to be contributing to the development of stent thrombosis in our patient. He remained on prasugrel for the following 4–6 months without incident. These results indicate complete resistance to the antiplatelet effect of clopidogrel, an effect that was rescued with prasugrel treatment in our patient. Optimal diagnostic cut-off measures used here are congruent with previous reports utilizing the VerifyNow system.7,10
Discussion
Indeed, the most potent predictor of drug-eluting ST (in the absence of procedural failure) is early discontinuation of antiplatelet therapy.1,2 However, even in the most compliant patients, ST still occurs as a result of high residual platelet reactivity owing, ostensibly, to variability in degrees of platelet responsiveness to thienopyridine inhibition.8 Accordingly, trials looking both retrospectively and prospectively at the effects of platelet reactivity following PCI have demonstrated strong correlations between a poor response to the antiplatelet effects of thienopyridines (referred to as high ‘on-treatment’ platelet reactivity) and drug-eluting ST, thus confirming that platelet reactivity is a powerful predictor of adverse clinical outcomes following PCI.11,12 Here, we highlight the deleterious effects of complete platelet unresponsiveness to clopidogrel in our patient who underwent an event-free index PCI with IVUS confirmation of procedural success.
Cases such as ours have been the driving force behind the development of new clinical tools aimed at rapidly and efficiently assessing platelet unresponsiveness to thienopyridine treatment or, in our case, so-called ‘clopidogrel resistance.’ Clinical assessment of platelet responsiveness to thienopyridines has been accomplished routinely in the laboratory through the use of light transmittance aggregometry (LTA), a technique based on light transmittance detection following ADP stimulation of P2Y12-activated platelet aggregation in platelet-rich plasma. Although LTA remains the criterion standard for evaluating platelet function, there are caveats to the use of LTA, including the fact that it is both time and labor intensive and not universally available, all of which make its standardized use problematic. Newer point-of-care platelet reactivity tests are becoming available that allow for rapid detection of platelet function, and a few tests have shown good correlations with LTA.5,13 However, currently the only point-of-care assays shown to predict clinical outcomes based on platelet reactivity assessment are the VerifyNow P2Y12 rapid analyzer (Accumetrics, Inc.) and the Plateletworks assay (Helena Laboratories, Beaumont, Texas).7,10
The VerifyNow assay, utilized in our patient, has been well-validated in prospective clinical trials aimed at assessing the ability of this rapid analyzer to predict clinical outcomes based on quantitation of on-treatment platelet reactivity in patients undergoing elective7 and urgent/emergent14 drug-eluting coronary artery stent placement.7 Its efficacy has been further substantiated in the largest prospective clinical trial to date, POPULAR (Do Platelet Function Assays Predict Clinical Outcomes in Clopidogrel-Pretreated Patients Undergoing Elective PCI), which compared virtually all available platelet reactivity testing modalities to the accepted gold standard, LTA.10 Concordant with previous reports that identified a PRU range of 174–291 and an optimal cut-off value of > 235 to predict post-discharge outcomes at 6 months [negative predictive value (NPV) of 99% (95% CI: 98–100)],7 the POPULAR investigators determined that a PRU cut-off value of > 236 had NPV of 94.3% in predicting an atherothrombotic event after PCI (versus 93.8% NPV for the gold standard LTA). Thus, two completely separate studies performed by different investigators found a virtually identical optimal diagnostic cut-off measure able to discriminate patients who suffered atherothrombotic events following PCI with drug-eluting stents from those who did not.
Our patient, with on-treatment PRU values of 429 and 454 over 2 consecutive days, respectively, meets not only the high on-treatment non-responder criteria, but in fact shows no measurable platelet inhibition in response to optimal clopidogrel therapy (Table 1). Is there a therapeutic alternative for these patients? Evidence exists detailing the putative dose-dependent effects of clopidogrel in poor responders. In this study, patients with high on-treatment platelet reactivity determined by LTA and a suboptimal response to clopidogrel received a favorable antiplatelet effect by increasing the dosing regimen from 75 mg per day to 150 mg per day.15 However, this regimen would have had no effect in our patient with a complete absence of response to the antiplatelet effects of clopidogrel. Therefore, given its recent Food and Drug Administration (FDA) approval and based on compelling data from the TRITON TIMI 38 investigators,16,17 we chose to substitute clopidogrel for the new thienopyridine agent prasugrel with favorable results (76% platelet inhibition within 60 minutes of prasugrel loading; Table 1) and prevention of stent thrombosis recurrence.
The differences between prasugrel and clopidogrel are thought to be pharmacodynamic, pharmacokinetic and genetic. Both begin as prodrugs requiring bioactivation via the hepatic cytochrome P450 (CYP450) system to their active metabolite before noncompetitively inhibiting the platelet P2Y12 ADP receptor and producing their irreversible antiplatelet effects on platelets.8 However, whereas clopidogrel requires a two-step, CYP450-dependent conversion to its active metabolite, prasugrel only requires a single-step activation; this increases prasugrel’s bioavailability relative to clopidogrel, with an apparent enhancement of its pharmacologic potency and less response variability with respect to platelet inhibitory activity as compared to clopidogrel. Indeed, our patient experienced almost 80% platelet inhibition within 60 minutes of administration of prasugrel, similar to that described by Wiviott and the TIMI 38 investigators.18 Additionally, mutations in certain CYP genes result in reduced function alleles that have been shown to significantly affect the pharmacokinetics and pharmacodynamics of clopidogrel and lead to higher rates of major adverse cardiovascular events, including stent thrombosis. On the contrary, these CYP mutations appear to have no effect on prasugrel metabolism and are inconsequential to the clinical outcomes surrounding its use.19 The most frequent CYP mutations are point mutations in the CYP2C19 gene, resulting in reduced exposure to the active thienopyridine metabolite, and thus, less platelet inhibition. These genetic polymorphisms, found predominately in patients such as ours originating from South Asia, are responsible for 99% of poor metabolizers and significantly diminish both the pharmacokinetic and pharmacodynamic responses to clopidogrel.19 Unfortunately, our patient declined genetic testing; as such, we can only speculate as to whether genetically determined perturbations in drug metabolism contributed to his presentation. We were, however, able to confirm the absence of obvious underlying acquired and/or genetic thrombophilic states contributing to his coronary atherothrombotic event (Table 2), making his ethnicity a more likely contributor to ST.
The exact mechanistic advantages of prasugrel over clopidogrel require further investigation and we await prospective trials evaluating its efficacy when substituted for clopidogrel in patients such as ours. Nevertheless, prasugrel’s clinical superiority, somewhat offset by increased bleeding risk, has been clearly demonstrated in patients undergoing coronary artery stenting when compared to clopidogrel.16–18 Given the potentially fatal repercussions arising from high on-treatment platelet reactivity following PCI, perhaps an alternative to current conventional practice (i.e., platelet function testing after ST has occurred) would be to initiate rapid examination of platelet reactivity prior to stent placement in the catheterization lab. Or perhaps a more prudent approach would involve measurement of on-treatment platelet reactivity in genetically predisposed carriers of reduced-function CYP mutations and/or those with high-risk lesions or lesions with a higher anatomic risk such as ostial and proximal LAD and left main lesions. Prasugrel’s recent FDA approval provides clinicians within the United States with a viable alternative to clopidogrel in patients with high on-treatment platelet reactivity or complete resistance and may help to reduce the threat of atherothrombosis and ST in patients following PCI.
In summary, this presentation underscores a potentially lethal consequence of insufficient thienopyridine-mediated platelet inhibition (clopidogrel resistance) following PCI. Although diagnosis of clopidogrel resistance was delayed in our patient, we hope that our case emphasizes the importance of rapid point-of-care assays in the early detection of non-responders, as implementation of an effective therapeutic alternative is paramount in these patients. Additionally, we hope that our discussion regarding the multifactorial nature of clopidogrel resistance offers some guidance as to how to approach patients such as ours and, since no consensus exists for the management of clopidogrel resistance, we hope that our success with prasugrel attests to the efficacy of this strategy in appropriate clinical scenarios.
References
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005;293:2126–2130.
- Motovska Z, Widimsky P, Marinov I, et al. Clopidogrel resistance “live” — The risk of stent thrombosis should be evaluated before procedures. Thromb J 2009;7:6.
- Bavry AA, Kumbhani DJ, Helton TJ, et al. Late thrombosis of drug-eluting stents: A meta-analysis of randomized clinical trials. Am J Med 2006;119:1056–1061.
- Cutlip DE, Baim DS, Ho KK, et al. Stent thrombosis in the modern era: A pooled analysis of multicenter coronary stent clinical trials. Circulation 2001;103:1967–1971.
- Moerenhout CM, Claeys MJ, Haine S, et al. Clinical relevance of clopidogrel unresponsiveness during elective coronary stenting: Experience with the point-of-care platelet function assay-100 C/ADP. Am Heart J 2010;159:434–438.
- Wiviott SD, Antman EM, Winters KJ, et al. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: Results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 trial. Circulation 2005;111:3366–3373.
- Price MJ, Endemann S, Gollapudi RR, et al. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J 2008;29:992–1000.
- Raju NC, Eikelboom JW, Hirsh J. Platelet ADP-receptor antagonists for cardiovascular disease: Past, present and future. Nat Clin Pract Cardiovasc Med 2008;5:766–780.
- Malinin A, Pokov A, Spergling M, et al. Monitoring platelet inhibition after clopidogrel with the VerifyNow-P2Y12(R) rapid analyzer: The VERIfy Thrombosis risk ASsessment (VERITAS) study. Thromb Res 2007;119:277–284.
- Breet NJ, van Werkum JW, Bouman HJ, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA 2010;303:754–762.
- Buonamici P, Marcucci R, Migliorini A, et al. Impact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosis. J Am Coll Cardiol 2007;49:2312–2317.
- Gori AM, Marcucci R, Migliorini A, et al. Incidence and clinical impact of dual nonresponsiveness to aspirin and clopidogrel in patients with drug-eluting stents. J Am Coll Cardiol 2008;52:734–739.
- Van der Planken MG, Claeys MJ, Vertessen FJ, et al. Comparison of turbidimetric aggregation and in vitro bleeding time (PFA-100) for monitoring the platelet inhibitory profile of antiplatelet agents in patients undergoing stent implantation. Thromb Res 2003;111:159–164.
- Marcucci R, Gori AM, Paniccia R, et al. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: A 12-month follow-up. Circulation 2009;119:237–242.
- Angiolillo DJ, Shoemaker SB, Desai B, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: Results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation 2007;115:708–716.
- Montalescot G, Wiviott SD, Braunwald E, et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): Double-blind, randomized controlled trial. Lancet 2009;373:723–731.
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–2015.
- Wiviott SD, Trenk D, Frelinger AL, et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: The Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007;116:2923–2932.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: Relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation 2009;119:2553–2560.
_________________________________________
From the *Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, Maryland and †the Division of Cardiovascular Medicine, University of Louisville School of Medicine, Louisville, Kentucky.
The authors report no conflicts of interest regarding the content herein.
Manuscript submitted November 5, 2010, provisional acceptance given November 29, 2010, final version accepted December 13, 2010.
Address for correspondence: Jeffrey J. Rade, MD, The Johns Hopkins School of Medicine, Division of Cardiology, Blalock 524 — Room 501, 600 North Wolfe Street, Baltimore, MD 21287. Email: jjrade@jhmi.edu











