Single-Center Experience of Catheter Ablation for Atrial Fibrillation Using Multi-Electrode Mapping and Ablation Catheters
Abstract: Purpose. Radiofrequency ablation (RFA) is an established therapy for the treatment of paroxysmal and persistent atrial fibrillation (AF). Many techniques have been reported to achieve RFA. We report a single-center experience of RFA using three multi-electrode catheters. Methods. We collected data of the patients who had RFA for AF using custom-designed multi-electrode mapping and ablation catheters between May 2007 and November 2009 at this center. Results. A total of 105 patients aged 56 ± 9.6 years underwent RFA using three multi-electrode catheters. Eighty-seven patients were new and 18 patients had re-do AF ablation using the multi-electrode mapping and ablation catheters. In the new patients, the mean procedure duration was 141 ± 38 minutes and fluoroscopy time was 38 ± 4 minutes. The mean duration of follow-up was 15.8 ± 6.4 months. Symptomatic improvement was achieved in 75 patients (86%), 48 patients (55%) remained in sinus rhythm (SR) after the first procedure, while 7 (8%) had multiple procedures and remained in SR without antiarrhythmic drugs (AAD). Fourteen patients (16%) required AAD following a single procedure and 1 patient (1.1%) after multiple procedures to remain in SR. Seven patients (8%) had reduced burden of symptoms. No improvement occurred in 12 patients (13.7%). In the 18 re-do patients, 15 (83.3%) had symptom improvement. Four patients (22.2%) remained in SR after a single procedure and 4 patients (22.2%) required multiple procedures to remain in SR without AAD. One patient (5.5%) remained in SR on AAD following a single procedure and 1 patient (5.5%) remained in SR on AAD following multiple procedures. Five patients (27%) had reduced burden of symptoms and 3 patients (16.6%) had no improvement. Conclusion. PVI using multi-electrode mapping and ablation catheters is an effective treatment of paroxysmal and persistent AF with a complication rate equivalent to published data.
J INVASIVE CARDIOL 2011;23(10):407–413
Key words: atrial fibrillation, percutaneous endocardial ablation, complications, PVI, success rate
____________________________________________
Atrial fibrillation (AF) is a major health problem.1 The clinical consequences range from increased hospitalization, diminished quality of life, and increase in congestive heart failure, to devastating thromboembolic events and increase in mortality.2-6 Radiofrequency ablation (RFA) is an established therapy for selected patients with paroxysmal and persistent AF.7 The cornerstone of RFA for AF is the electrical isolation of the pulmonary veins (PVI), though in selected patients additional non-pulmonary vein lesions are performed.8 Traditionally, PVI is achieved using an irrigated single-tip catheter placing contiguous point-by-point lesions either targeting the electrical connections between the left atrium (segmental PVI) or by placing continuous circular lesions around the pulmonary veins (wide area circumferential ablation; WACA).9-11
However, such procedures can be technically complex and are associated with long fluoroscopy and procedure times. Recently, anatomically designed multi-electrode mapping and ablation catheters have been introduced in order to reduce procedure and fluoroscopy times and potentially simplify the procedure (Medtronic Ablation Frontiers, Medtronic, Inc.).12 The long-term efficacy of these catheters in comparison to a single-tip catheter-based approach is unknown and is currently under investigation by randomized trials.13 The aim of this single-center, retrospective, observational study was to analyze the safety and efficacy of the three multi-electrode catheters.
Methods
Population. Southampton University Hospital is a regional cardiac electrophysiology center for the Central South Coast Cardiac Network. We included consecutive patients who had undergone RFA for persistent or paroxysmal AF (May 2007 and November 2009) with an ablation strategy utilizing a circular catheter for pulmonary vein isolation (PVAC; Medtronic Ablation Frontiers), as well as custom-designed catheters for ablation of complex fractionated atrial electrograms (CFAE) on the septum and body of the left atrium.
Paroxysmal atrial fibrillation (PAF) was defined as recurrent AF (≥2 episodes) that terminated spontaneously within 7 days. Persistent AF was defined as AF that sustained for longer than 7 days, or that required electrical or chemical cardioversion.14,15
Patient selection. At present, there are no randomized clinical trials demonstrating improved survival or stroke reduction with RFA in AF, and therefore the procedure is predominantly reserved for symptomatic patients to improve quality of life. National and international guidelines advocate RFA to relieve symptoms in selected AF patients who are symptomatic despite anti-arrhythmic drugs (AAD) or who are intolerant to medication due to side effects.14,15 In our study, patients were selected for RFA consistent with these guidelines.
Preprocedure anticoagulation. All patients were anticoagulated with warfarin for a minimum period of 4 weeks preprocedure with a target INR of 2.0–3.0.16
 Multi-electrode catheters and RF generator. The PVAC is a 9 Fr, over-the-wire, decapolar mapping and ablation catheter with an adjustable 25 mm diameter array at the distal portion (Figure 1A). The platinum electrodes are 3 mm long with 3 mm spacing. The shaft of the catheter can be deflected in both directions, facilitating correct positioning at each pulmonary vein antrum. Furthermore, the electrode array can be extended to assume a spiral configuration to enable different tissue contact and enhance mapping inside the PV. In addition, two other specifically designed multi-electrode catheters were used to target left atrial CFAE. The multi-array septal catheter (MASC; Medtronic Ablation Frontiers) is designed to target CFAE on the intra-atrial septum (Figure 1B), and the multi-array ablation catheter (MAAC; Medtronic Ablation Frontiers) CFAE in the left atrial body (Figure 1C).
Multi-electrode catheters and RF generator. The PVAC is a 9 Fr, over-the-wire, decapolar mapping and ablation catheter with an adjustable 25 mm diameter array at the distal portion (Figure 1A). The platinum electrodes are 3 mm long with 3 mm spacing. The shaft of the catheter can be deflected in both directions, facilitating correct positioning at each pulmonary vein antrum. Furthermore, the electrode array can be extended to assume a spiral configuration to enable different tissue contact and enhance mapping inside the PV. In addition, two other specifically designed multi-electrode catheters were used to target left atrial CFAE. The multi-array septal catheter (MASC; Medtronic Ablation Frontiers) is designed to target CFAE on the intra-atrial septum (Figure 1B), and the multi-array ablation catheter (MAAC; Medtronic Ablation Frontiers) CFAE in the left atrial body (Figure 1C).
The GENius multi-channel RF Ablation Generator (Medtronic, Inc.) is capable of independently delivering radiofrequency energy to a maximum of 12 electrodes simultaneously. Power can be delivered as unipolar energy, with current flowing from the catheter electrode to the dispersive electrodes on the patient’s back, or bipolar energy, with current flowing between two adjacent selected pairs of electrodes at the catheter array. The generator has 5 preset energy settings: bipolar, unipolar, and 3 ratios of bipolar/unipolar energy (4:1, 2:1, and 1:1).20 Preclinical studies have demonstrated that lesion depth is greater with energy settings with a greater proportion of unipolar energy, with lesion depths of up to 7 mm achievable.20 During RF application, energy delivery to individual electrodes is modulated to reach a user-defined target temperature.
Ablation procedure. A preprocedure cardiac MRI or CT scan was obtained in all patients to detect any anatomical anomalies as well as define pulmonary vein anatomy.17,18 In all cases, a preprocedural transesophageal echocardiogram was performed to look for left atrial thrombus.19
Procedures were performed in the fasting state under conscious sedation with local anesthesia. The ablation technique is described in detail elsewhere.20 In brief, vascular access was via the right femoral vein, right subclavian vein, and right femoral artery. A quadripolar catheter was placed in the coronary sinus (CS) for pacing and recording electrograms. A single transseptal puncture was performed with a Brockenbrough (BRK; Daig Corporation) or RF needle (NRG™ RF needle; Baylis Medical, Inc.) guided by fluoroscopy and contrast injection, and a 12.5 Fr steerable sheath (Channel; Bard Corporation) placed in the left atrium. Following transseptal puncture, systemic anticoagulation was achieved with a loading dose of 100 IU/kg intravenous unfractionated heparin followed by an infusion of 10 IU/kg/hour. During the procedure, the activated clotting time (ACT) was measured every 30 minutes and the heparin infusion rate was adjusted to maintain an ACT in the target range of 300–350 seconds.15
Prior to ablation, contrast angiography was used to define the PV as a reference for positioning the PVAC catheter. In all patients, isolation of PV was performed using the PVAC catheter. The steerable sheath was used to aid positioning of the PVAC catheter and a 0.032˝ guidewire was introduced into the targeted PV. The PVAC was then introduced into the antrum of the PV using an over-the-wire technique. In this study, RF energy was typically delivered to all electrode pairs for up to 60 seconds per application, using a 4:1 bipolar/unipolar energy ratio and a target temperature of 60 ˚C. The catheter was then rotated and further RF energy delivered if necessary. For potentials that were persistently detectable after ablation using a 4:1 energy ratio, further ablation using a 2:1 ratio was performed to achieve greater lesion depth.
Additional ablation of spontaneous CFAE with the MAAC and MASC catheters was performed in selected patients. CFAE were defined as electrograms with a cycle length ≤120 ms or shorter than the AF cycle length in the coronary sinus, or electrograms that were fractionated or displayed continuous electrical activity.8 Ablation of CFAE was performed in all patients with persistent AF and selected patients with PAF, especially those undergoing a re-do procedure. In patients who were in SR, AF was induced with burst atrial pacing in order to perform CFAE ablation.
The intraprocedural endpoints for PV isolation were entrance block into the PV during SR or CS pacing, and exit block during pacing from the PVAC in the PV distal to the ablation line. The CFAE was ablated until local activity was no longer observed. At the completion of the procedure, IV heparin was discontinued and sheaths were removed when the ACT was <160 seconds.15
Postablation management. All patients were monitored for 24 hours as an inpatient prior to discharge. Unless there were complications, warfarin was routinely restarted on the day of the procedure with subcutaneous low molecular weight heparin cover until the INR was ≥2. All patients were continued on an AAD for 6 weeks postablation and warfarin was continued (target INR, 2–3) until clinical follow-up.22
Follow-up, monitoring, and outcome measures. All patients were followed at our institution in an outpatient clinic at 3–6 months postprocedure. At follow-up, all patients had a 12-lead electrocardiogram (ECG) and were clinically assessed to evaluate their symptom burden. Twenty-four hour and 7-day rhythm monitoring was performed in selected patients depending on symptomatic status.21
The patients’ symptomatic responses to the ablation procedure were made through a detailed clinical interview during their outpatient clinic appointment and were based on the subjective perception of the patients’ own symptoms, as well as their frequency, duration, and impact on their daily activities and lifestyle. When performed, rhythm monitoring was analyzed for the absence, duration, and frequency of AF. The patients’ symptomatic responses to the procedure were classed as “no improvement,” “reduced AF burden,” or “complete resolution.” Patients who had a recurrence of symptoms consistent with AF or documented AF on rhythm monitoring at their first clinic review were continued or reinitiated on an AAD and then reassessed after 3–6 months. Patients who remained symptomatic were then considered for repeat AF ablation.
For the purposes of the study results, patients were classified as in “sinus rhythm” if they had complete resolution of their AF symptoms at last follow-up, as well as no documented episodes of AF lasting 30 seconds or more after a blanking period of 3 months postprocedure if rhythm monitoring was performed.7,21 Patients who required continued AAD use to achieve symptom resolution, and no evidence of AF on rhythm monitoring as described above, were classified as “sinus rhythm with AAD.” Patients who remained symptomatic with or without an AAD, but whose symptoms improved postablation, were classified as “reduced AF burden.” Patients who experienced no symptomatic improvement postablation were classified as a “failure.”
Statistical analysis. The data from this single-center experience are presented using descriptive statistics.
Results
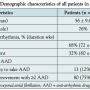 Patient characteristics. During the study period, a total of 105 patients underwent AF ablation using multi-electrode mapping and ablation catheters (76% male; mean age, 56 ± 9.6 years). Eighty-seven patients were undergoing ablation for the first time, while 18 had a previous AF ablation. Seventy-two patients (68.5%) had paroxysmal AF, with a mean duration of 16.7 ± 16.9 months, and 33 patients (31.4%) had persistent AF, with a mean duration of 15 ± 17.2 months. The mean follow-up duration after ablation was 16.7 ± 7.4 months (range, 6–36 months). Patient characteristics are shown in Table 1.
Patient characteristics. During the study period, a total of 105 patients underwent AF ablation using multi-electrode mapping and ablation catheters (76% male; mean age, 56 ± 9.6 years). Eighty-seven patients were undergoing ablation for the first time, while 18 had a previous AF ablation. Seventy-two patients (68.5%) had paroxysmal AF, with a mean duration of 16.7 ± 16.9 months, and 33 patients (31.4%) had persistent AF, with a mean duration of 15 ± 17.2 months. The mean follow-up duration after ablation was 16.7 ± 7.4 months (range, 6–36 months). Patient characteristics are shown in Table 1.
Procedure details. In 63 patients (60%), PVI alone was performed using the PVAC catheter. In 42 patients (40%), CFAE were additionally targeted using the MAAC catheter in 34 patients (32%) and the MASC catheter in 19 patients (18%). Mean procedure time was 146 ± 44 minutes and mean fluoroscopy time was 39 ± 14 minutes.
Procedure Outcome
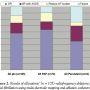 Overall result. The overall results are shown in Table 2 and Figure 2. The time to recurrence of AF is shown in Figure 3. Symptomatic improvement was achieved in 90 patients (85.7%), 52 patients (49.5%) remained asymptomatic and in SR at last follow-up after first procedure while 9 (8.5%) had multiple procedures and remained in SR without AAD. AAD were required in 15 patients (14.2%) following a single procedure and for 2 patients (1.9%) after multiple procedures to remain in SR. Twelve patients (11.4%) had reduced burden of symptoms. No improvement occurred in 15 patients (14.2%). The mean number of procedures performed per patient was 1.12.
Overall result. The overall results are shown in Table 2 and Figure 2. The time to recurrence of AF is shown in Figure 3. Symptomatic improvement was achieved in 90 patients (85.7%), 52 patients (49.5%) remained asymptomatic and in SR at last follow-up after first procedure while 9 (8.5%) had multiple procedures and remained in SR without AAD. AAD were required in 15 patients (14.2%) following a single procedure and for 2 patients (1.9%) after multiple procedures to remain in SR. Twelve patients (11.4%) had reduced burden of symptoms. No improvement occurred in 15 patients (14.2%). The mean number of procedures performed per patient was 1.12.
 Paroxysmal AF. Seventy-two patients had PAF. Symptomatic improvement was achieved in 62 patients (90%), 39 patients (54%) remained asymptomatic and in SR at last follow-up after first procedure while 7 (9.7%) had multiple procedures and remained in SR without AAD. AAD were required in 8 patients (11%) following a single procedure and for 1 patient (1.3%) after multiple procedures to remain in SR. Ten patients (13.8%) had reduced burden of symptoms. No improvement occurred in 7 patients (9.7%). The mean number of procedures performed per patient was 1.12. The comparative results of PAF and persistent AF are shown in Figure 2.
Paroxysmal AF. Seventy-two patients had PAF. Symptomatic improvement was achieved in 62 patients (90%), 39 patients (54%) remained asymptomatic and in SR at last follow-up after first procedure while 7 (9.7%) had multiple procedures and remained in SR without AAD. AAD were required in 8 patients (11%) following a single procedure and for 1 patient (1.3%) after multiple procedures to remain in SR. Ten patients (13.8%) had reduced burden of symptoms. No improvement occurred in 7 patients (9.7%). The mean number of procedures performed per patient was 1.12. The comparative results of PAF and persistent AF are shown in Figure 2.
 PAF ablation with PVAC only. PVAC catheter for only PVI was used in 50 PAF patients (69.4%). Forty-six patients (92%) had symptomatic improvement. Thirty patients (60%) remained in SR without AAD, while 6 patients (18%) required drugs to stay in SR after a single procedure. Three patients (9%) had multiple procedures and remained in SR without drugs and 1 patient required AAD after multiple procedures to stay in SR. Six patients (18%) had reduced burden of symptoms. The procedure was unsuccessful in 4 patients (12%) both to maintain SR and improve symptoms.
PAF ablation with PVAC only. PVAC catheter for only PVI was used in 50 PAF patients (69.4%). Forty-six patients (92%) had symptomatic improvement. Thirty patients (60%) remained in SR without AAD, while 6 patients (18%) required drugs to stay in SR after a single procedure. Three patients (9%) had multiple procedures and remained in SR without drugs and 1 patient required AAD after multiple procedures to stay in SR. Six patients (18%) had reduced burden of symptoms. The procedure was unsuccessful in 4 patients (12%) both to maintain SR and improve symptoms.
Combined PVAC and CFAE ablation for PAF. CFAE ablation as an adjunct was used in 21 PAF patients (29%). Symptomatic improvement occurred in 18 patients (85.7%). Eight patients (38%) remained in SR without AAD and 2 patients (9.5%) required drugs to remain in SR after a single procedure. Four patients (19%) required multiple procedures to stay in SR without AAD. Four patients (19%) had reduced burden of symptoms. No success was achieved in 3 patients (14.2%). The comparative results of PVI only and CFAE as adjunct for treatment of PAF are shown in Figure 4.
Persistent AF. Thirty-three out of 105 patients were known to have persistent AF. Symptomatic improvement was achieved in 25 patients (75.7%), 13 patients (39.3%) remained in SR after the first procedure while 2 patients (6%) had multiple procedures and remained in SR without AAD. AADs were required in 7 patients (21%) following a single procedure and for 1 patient (3%) after multiple procedures to remain in SR. Two patients (6%) had reduced burden of symptoms. No improvement occurred in 8 patients (24.2%). The mean number of procedures performed per patient was 1.12.
Persistent AF ablation with PVAC only. PVAC catheter for only PVI was used in 12 persistent AF patients (36.3%). Nine patients (75%) had symptomatic improvement. Seven patients (58.3%) remained in SR without AAD, while 1 patient (8.3%) required drugs to stay in SR after a single procedure. One patient (8.3%) had multiple procedures and remained in SR without drugs. The procedure was unsuccessful in 3 patients (25%) both to maintain SR and improve symptoms.
 Combined PVAC and CFAE ablation for persistent AF. CFAE ablation as an adjunct was used in 21 persistent AF patients (63.6%). Symptomatic improvement occurred in 16 patients (76.1%). Six patients (28.5%) remained in SR without AAD and 6 patients (28.5%) required drugs to remain in SR after a single procedure. One patient (4.7%) required multiple procedures to stay in SR without AAD and 1 patient (4.7%) required multiple procedures and AAD to stay in SR. Two patients (9.5%) had reduced burden of symptoms. No success was achieved in 5 patients (24%). The comparative results of PVI only and CFAE as adjunct for treatment of persistent AF are shown in Figure 4.
Combined PVAC and CFAE ablation for persistent AF. CFAE ablation as an adjunct was used in 21 persistent AF patients (63.6%). Symptomatic improvement occurred in 16 patients (76.1%). Six patients (28.5%) remained in SR without AAD and 6 patients (28.5%) required drugs to remain in SR after a single procedure. One patient (4.7%) required multiple procedures to stay in SR without AAD and 1 patient (4.7%) required multiple procedures and AAD to stay in SR. Two patients (9.5%) had reduced burden of symptoms. No success was achieved in 5 patients (24%). The comparative results of PVI only and CFAE as adjunct for treatment of persistent AF are shown in Figure 4.
Primary Procedures (New Patients)
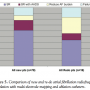 In 87 patients, the multi-electrode mapping and ablation catheters were used for the first time. Among them, 62 patients (71%) had diagnosis of PAF and 25 patients (28.7%) had persistent AF. The mean duration of symptoms was 14.1 ± 14.8 months. The mean fluoroscopy time was 38 ± 14 minutes and duration of procedure was 141 ± 38 minutes. The mean duration of follow-up was 15.8 ± 6.4 months. The mean number of procedures performed per patient was 1.19. Symptomatic improvement was achieved in 75 patients (86%), 48 patients (55%) remained in SR after the first procedure while 7 patients (8%) had multiple procedures and remained in SR without AAD. Fourteen patients (16%) required AAD following a single procedure and 1 patient (1.1%) after multiple procedures to remain in SR. Seven patients (8%) had reduced burden of symptoms. No improvement occurred in 12 patients (13.7%). The comparative results of new and re-do patients are shown in Figure 5.
In 87 patients, the multi-electrode mapping and ablation catheters were used for the first time. Among them, 62 patients (71%) had diagnosis of PAF and 25 patients (28.7%) had persistent AF. The mean duration of symptoms was 14.1 ± 14.8 months. The mean fluoroscopy time was 38 ± 14 minutes and duration of procedure was 141 ± 38 minutes. The mean duration of follow-up was 15.8 ± 6.4 months. The mean number of procedures performed per patient was 1.19. Symptomatic improvement was achieved in 75 patients (86%), 48 patients (55%) remained in SR after the first procedure while 7 patients (8%) had multiple procedures and remained in SR without AAD. Fourteen patients (16%) required AAD following a single procedure and 1 patient (1.1%) after multiple procedures to remain in SR. Seven patients (8%) had reduced burden of symptoms. No improvement occurred in 12 patients (13.7%). The comparative results of new and re-do patients are shown in Figure 5.
Re-Do Procedures
Eighteen patients had re-do RFA with the multi-electrode mapping and ablation catheters. Fifteen patients (83.3%) had symptom improvement. Four patients (22.2%) remained in SR after a single procedure and 4 patients (22.2%) required multiple procedures to remain in SR without AAD. One patient (5.5%) remained in SR on AAD following a single procedure and 1 patient (5.5%) remained in SR on AAD following multiple procedures. Five patients (27%) had reduced burden of symptoms and 3 patients (16.6%) had no improvement.
Complications
Overall, 12 patients (11%) experienced a procedure-related complication. This comprised 2 strokes, 1 patient with pulmonary vein stenosis, 1 patient with symptomatic right phrenic nerve palsy, 1 cardiac tamponade requiring pericardiocentesis, and 6 femoral hematomas delaying hospital discharge.
Both patients who suffered strokes presented with mild dysphasia and right hand in-coordination. One patient had PVI alone, but had a previous history of a transient ischemic attack, while the other patient had additional CFAE ablation. In both cases, the diagnosis was confirmed with a brain CT that demonstrated subacute left middle cerebral artery infarction. In 1 patient, the symptoms resolved spontaneously within 24 hours of onset. The other patient had minor residual in-coordination and slurred speech associated with physical and mental fatigue at 6 months postprocedure.
One patient suffered stenosis of both left superior and inferior pulmonary veins which presented 7 months after the procedure as exertional breathlessness. The diagnosis was made using cardiac MRI. The preprocedural MRI demonstrated unremarkable pulmonary vein anatomy with normal-sized left-sided veins. In view of the symptoms and severity of the stenosis, both veins were treated with successful balloon dilatation.
One patient experienced right phrenic nerve palsy. It was unclear whether this occurred during ablation of the right superior or inferior PV.
Discussion
The multi-electrode mapping and ablation catheters used in this study do not require a complex mapping system; they are designed as three distinct shapes according to anatomical utilization. These catheters have the ability to both map and ablate through multiple electrodes, thus potentially reducing procedure time for AF ablation. Previous studies have demonstrated that the results of AF ablation using these multi-electrode catheters are comparable to those using conventional single-tip ablation catheter methods.12,20,23-25
Our results are consistent with those from previous studies, and demonstrate that AF ablation using these multi-electrode catheters is effective and relatively safe for the treatment of paroxysmal as well as persistent AF. In addition, the procedure and fluoroscopy times in our study are lower compared to published series using conventional single-tip catheters. In our study, symptomatic improvement was observed in 86% of patients who had undergone a first AF ablation procedure and in 83% of patients undergoing a re-do procedure. After first procedure with multi-electrode mapping and ablation catheters, 55% of patients remained in SR while only 22% of patients remained in SR after first re-do RFA with these catheters. Similarly, symptomatic improvement was achieved in 90% of patients with PAF and 54% remained in SR after the first procedure, but for persistent AF, symptomatic improvement was achieved in 75.7% of patients, and only 39.3% of patients remained in SR after first procedure. These results suggest that RFA for AF with the anatomically designed catheters is more favorable in PAF patients and in those who are undergoing ablation for the first time.
Previous observational studies of the efficacy of multi-electrode mapping and ablation catheters in the treatment of PAF reported arrhythmia-free survival rates of between 79% and 86%.20,23-25 A randomized study of WACA (51 pts) and PVAC catheter (51 pts) RFA for PAF reported 71% and 77% success rates at 6 months, respectively, confirming the effectiveness of the PVAC catheter in patients with PAF.12 In our study, the lower success rate of arrhythmia-free survival is most likely a reflection of the mixed patient population, with 32% of patients having persistent AF, as well as a relatively long duration of follow-up as a rapid increase in the recurrence of AF that was noticed after 6 months of the index procedure in the previous studies. Additionally, the patients’ symptom statuses were not reported in the above studies and the results were based on ambulatory monitoring, whereas the limitation of monitoring in patients with PAF is well established.21 The indication for AF ablation is predominantly driven by symptoms; therefore, our approach of symptom-driven monitoring may be more appropriate for documentation of recurrence than strict time-related monitoring as performed in the above studies at 1 month and 6 months. In our study, monitoring was performed according to the patients’ symptoms and they were declared free of arrhythmia if no AF of more than 30-second duration was documented at the time of their symptoms.
The results of studies investigating the incremental benefit of performing CFAE ablation in addition to PVI are conflicting.15,26 In our study, the addition of CFAE ablation to PVI did not show any improvement in the outcome, and rather conversely, the adjunctive use of CFAE ablation led to less favorable results both in persistent and PAF. However, this is likely to be a reflection of selection bias, as additional CFAE ablation was most likely to be performed in patients with more established AF whose outcomes are likely to be worse irrespective of the ablation strategy performed.
The complication rate in our study is comparable to previous reports.23-25 PV stenosis and phrenic nerve palsy are usually related to the applications of RFA inside the pulmonary veins,20 and the risk especially increases in PV with common ostium, which is frequently observed on the left side.27 Additionally, the circular PVAC catheter can be adapted to some extent by clockwise or anti-clockwise rotation to adjust according to the size of the vein, but is not considered a favorable catheter to ablate veins with common ostium. Previous studies have demonstrated that the incidence of PV stenosis was not reduced with the use of 3-dimensional left atrial mapping.27 PV stenosis and phrenic nerve palsy both occurred in this series in 2 separate patients; however, both of these patients had normal venous anatomy on cardiac MRI before RFA with PVAC.
Stroke is one of the major risks of AF ablation.23-25 Although the 2% stroke rate is similar to the published data, it is important to further evaluate this new technology, as one would speculate that using simultaneous long linear application without control of tissue contact may have higher risk of clot formation despite maintaining an ACT of between 300 and 350 s.
Previous observational studies have shown survival benefit from AF ablation; however, at present there are no published randomized studies demonstrating reduction in thromboembolic risk, especially stroke, and long-term survival benefit associated with successful AF ablation.28-30 Therefore, in our study, the primary outcome is based on symptomatic relief and reduction in the use of AAD.
Limitations. Our study has limitations. First, it is a small, non-randomized, single-approach, retrospective, single-center study, reporting outcomes in terms of symptomatic improvement and complications for AF ablation using multi-electrode ablation catheters. Second, our follow-up, at 16.7 ± 7.4 months, is relatively short. This is especially important when studying the long-term success rates and complications including pulmonary vein stenosis, where the incidence is known to be strongly dependent on duration of follow-up. Third, we have performed monitoring in only 55% of patients; monitoring of the remaining patients may have also diagnosed asymptomatic recurrence of PAF, lowering our success rates. However, the diagnosis of PAF and especially asymptomatic PAF is difficult even with conventional 24-hour and 7-day ECG monitoring; therefore, in our study, symptom-led monitoring was performed. Additionally, the consensus document from the Heart Rhythm Society on AF ablation emphasized the importance of symptomatic AF and that asymptomatic AF is a universal limitation.21,22 Fourth, the number of RF applications needed per vein was not systematically recorded at the time of ablation.
Conclusion
The results of AF ablation with multi-electrode mapping and ablation catheters are comparable to previously used techniques and associated with short procedure times.
Further multicenter randomized studies are required and currently underway to evaluate the efficacy of these catheters and its effect on survival and stroke prevention by comparison with previously used techniques including WACA and rhythm control strategies.
References
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285(18):2370–2375.
- Parikh SS, Jons C, McNitt S, Daubert JP, Schwarz KQ, Hall B. Predictive capability of left atrial size measured by CT, TEE, and TTE for recurrence of atrial fibrillation following radiofrequency catheter ablation. Pacing Clin Electrophysiol. 2010;33(5):532-540.
- Dorian P, Jung W, Newman D, et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000;36(4):1303-1309.
- Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–2925.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988.
- Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation. 1998;98(10):946–952.
- Terasawa T, Balk EM, Chung M, et al. Comparative effectiveness of radiofrequency catheter ablation for atrial fibrillation. Ann Intern Med. 2009;151(3):191-202.
- Oral H, Chugh A, Yoshida K, et al. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. J Am Coll Cardiol. 2009;53(9):782-789.
- Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–666.
- Pappone C, Oreto G, Lamberti F, et al. Catheter ablation of paroxysmal atrial fibrillation using a 3D mapping system. Circulation. 1999;100(11):1203–1208.
- Andrikopoulos G, Tzeis S, Vardas PE. Invasive therapy for atrial fibrillation: recent developments in ablation, navigation and mapping technology. Heart. 2011;97(3):237-243.
- Bulava A, Haniš J, Sitek D, et al. Catheter ablation for paroxysmal atrial fibrillation: a randomized comparison between multi-electrode catheter and point-by-point ablation. Pacing Clin Electrophysiol. 2010;33(9):1039–1046.
- University College London Hospitals, Southampton University Hospitals NHS Trust. Randomized trial of two ablation catheters in paroxysmal atrial fibrillation. https://clinicaltrials.gov/ct2/show/NCT00678340
- Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline). A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Heart Rhythm. 2011;8(1):157-176.
- Fuster V, Ryden LE, Cannom DS, et al; ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol. 2007;50(6):562.
- Blanc J, Almendral J, Brignole M, et al. Consensus document on antithrombotic therapy in the setting of electrophysiological procedures. Europace. 2008;10(5):513–527.
- Martinez M, Kirsch J, Syed I, et al. Utility of nongated multidetector computed tomography for detection of left atrial thrombus in patients undergoing catheter ablation of atrial fibrillation. JACC Cardiovasc Imaging. 2009;2(1):69-76.
- Ohyama H, Hosomi N, Takahashi T, et al. Comparison of magnetic resonance imaging and transoesophageal echocardiography in detection of thrombus in left atrial appendage. Stroke. 2003;34(10):2436–2439.
- Hayes CR, Keane D. Safety of atrial fibrillation ablation with novel multi-electrode array catheters on uninterrupted anticoagulation — a single-center experience. J Interv Card Electrophysiol. 2010;27(2):117-122.
- Boersma LV, Wijffels MC, Oral H, Wever EF, Morady F. Pulmonary vein isolation by duty-cycled bipolar and unipolar radiofrequency energy with a multielectrode ablation catheter. Heart Rhythm. 2008;5(12):1635–1642.
- Calkins H, Brugada J, Packer DL, et al. European Heart Rhythm Association (EHRA), European Cardiac Arrhythmia Society (ECAS), American College of Cardiology (ACC), American Heart Association (AHA), Society of Thoracic Surgeons (STS). HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4(6):816-861.
- Leong-Sit P, Roux JF, Zado E, et al. Antiarrhythmics after ablation of atrial fibrillation (5A study) six month follow-up study. Circ Arrhythm Electrophysiol. 2011 Feb;4(1):11-14. Epub 2010 Nov 13.
- Fredersdorf S, Weber S, Jilek C, et al. Safe and rapid isolation of pulmonary veins using a novel circular ablation catheter and duty-cycled RF generator. J Cardiovasc Electrophysiol. 2009;20(10):1097–1101.
- Wieczorek M, Hoeltgen R, Brueck M, Bandorski D, Akin E, Reza SA. Pulmonary vein isolation by duty-cycled bipolar and unipolar antrum ablation using a novel multielectrode ablation catheter system: first clinical results. J Interv Card Electrophysiol. 2009;27(1):23–31.
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3(1):32-38.
- Elayi CS, Verma A, Di Biase L, et al. Ablation for longstanding permanent atrial fibrillation: results from a randomized study comparing three different strategies. Heart Rhythm. 2008;5(12):1658-1664.
- Dong J, Vasamreddy CR, Jayam V, et al. Incidence and predictors of pulmonary vein stenosis following catheter ablation of atrial fibrillation using the anatomic pulmonary vein ablation approach: results from paired magnetic resonance imaging. J Cardiovasc Electrophysiol. 2005;16(8):845-852.
- Nademanee K, Schwab MC, Kosar EM, et al. Clinical outcomes of catheter substrate ablation for high-risk patients with atrial fibrillation. J Am Coll Cardiol. 2008;51(8):843-849.
- Hsu LF, Jais P, Sanders P, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351(23):2373-2383.
- Gentlesk PJ, Sauer WH, Gerstenfeld EP, et al. Reversal of the left ventricular dysfunction following ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18(1):9-14.
____________________________________________
From 1Wessex Cardiothoracic Unit, Department of Cardiology, Southampton University Hospitals NHS Trust, and 2Faculty of Medicine, University of Southampton, United Kingdom.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Zeb discloses that he has received educational grants and travel expenses from Medtronic UK and is on their speaker’s bureau. Dr. Yue discloses that he has received honoraria from Medtronic. He is a consultant to Medtronic and a member of their speaker’s bureau. Dr. Scott discloses that he has received educational grant(s) from Medtronic. He is a consultant to Medtronic, Boston Scientific and St. Jude, and a member of the Medtronic speaker’s bureau. Dr. Roberts discloses that he is a consultant to Medtronic, Boston Scientific and St. Jude, and a member of the Medtronic and St. Jude speaker’s bureaus. Dr. Morgan discloses that he has received honoraria from Medtronic. He is a member of the Medtronic, Boston Scientific and St. Jude speaker’s bureaus.
Manuscript submitted May 23, 2011, provisional acceptance given June 3, 2011, final version accepted August 17, 2011.
Address for correspondence: Dr. Mehmood Zeb, Clinical Cardiology Research Fellow, Wessex Cardiothoracic Unit, Department Cardiology, Southampton General Hospital, Tremona Road, Southampton, SO16 6YD. Email: mehmoodzeb@hotmail.com












