Segmental Diastolic Compression of Circumflex Coronary Artery Secondary to Pericardial Constriction: An Uncommon Cause of Angina Pectoris
ABSTRACT: Diastolic segmental compression of a native coronary artery is an uncommon cause of chest pain. Here we describe a 24-year-old woman with constrictive pericarditis who had retrosternal chest pain, progressive dyspnea, tachycardia, and bilateral leg edema. She was diagnosed with compression of the first and second obtuse marginal branches of left circumflex coronary artery secondary to constrictive pericarditis. She underwent pericardiectomy and her symptoms were relieved following surgery.
J INVASIVE CARDIOL 2012;24(5):E90-E92
Key words: constrictive pericarditis, coronary arteries, myocardial ischemia
__________________________________________
Constrictive pericarditis is a pathological condition characterized by local or global reduced pericardial compliance. Any process involving pericardial incision, inflammation, or calcification can cause this. Potential etiologies include cardiovascular surgery, radiation therapy, tuberculosis, fungal and viral diseases, and chronic renal failure.1,2 These patients usually present with dyspnea, fatigue, and lower extremity edema suggesting right sided heart failure. Diastolic compression of epicardial coronary artery secondary to constrictive pericarditis is extremely uncommon and results from external, inward pressure against an expanding myocardium during ventricular filling that leads to myocardial ischemia. This is distinct from myocardial bridge, a more common anomaly detected in 0.5%-16% of coronary angiograms,3 in which a portion of the epicardial coronary artery is embedded within the myocardium. Myocardial bridge can result in systolic compression of the vessel and myocardial ischemia.4
Compression of the native coronary arteries has also been described in patients with abnormal diastolic ventricular properties and reduced ventricular compliance, including aortic insufficiency,5-7 hypertrophic cardiomyopathy,8 and heart transplantation.9 In these conditions, the segmental diastolic coronary artery compression was primarily dependent on high intraventricular pressure. Although the diastolic compression of venous grafts secondary to constrictive pericarditis has been described previously,10 obstruction of the native coronary arteries secondary to this condition has never been reported. Here we describe a 24-year-old patient with constrictive pericarditis associated with segmental diastolic compression of native circumflex coronary artery. Her symptoms including angina pectoris improved significantly following pericardiectomy. To the best of our knowledge, the clinical case of constrictive pericarditis associated with segmental diastolic compression of native left circumflex coronary artery described here is the first case reported in the literature.
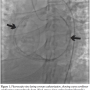 Case report. A 24-year-old woman was admitted to our hospital with progressive retrosternal chest pain, exertional dyspnea, tachycardia, and bilateral leg edema, persisting for 4 weeks. She had previous repair of coarctation of the aorta by left thoracotomy at age 13. Her blood pressure was 110/75 mm Hg, and her heart rate was 87 bpm. The physical examination was remarkable for mild jugular venous distension and pericardial knock. Liver function tests, electrolyte levels, cardiac enzymes, coagulation parameters, and urinalysis were within normal limits. Her ECG showed normal sinus rhythm, rightward axis, and intraventricular conduction delay. Chest x-ray revealed mediastinal enlargement with mild cardiomegaly, pericardial calcification, and right pleural effusion. Two-dimensional echocardiography demonstrated enhanced echogenicity of the pericardium suggesting the presence of calcification, biatrial enlargement, normal left ventricular dimensions, and systolic function (ejection fraction 59%), and abnormal ventricular septal motion. Cardiovascular magnetic resonance imaging confirmed pericardial thickening with calcification, presence of constrictive physiology with septal bounce, and abrupt cessation of left ventricle diastolic filling. Cardiac catheterization revealed equalization of end-diastolic pressures, and interdependence of ventricular pressures consistent with constrictive physiology. Coronary angiogram demonstrated the obliteration of the second obtuse marginal branch of left circumflex artery in systole and diastole, and compression of the first obtuse marginal branch that induced mid to late diastolic interruption of coronary flow (Video). The patient was referred to surgery and pericardiectomy was performed. Histopathologic examination of the pericardium excluded mycobacterium tuberculosis or granulomatous diseases. The patient was discharged home following surgery and her symptoms were improved during the postoperative follow-up.
Case report. A 24-year-old woman was admitted to our hospital with progressive retrosternal chest pain, exertional dyspnea, tachycardia, and bilateral leg edema, persisting for 4 weeks. She had previous repair of coarctation of the aorta by left thoracotomy at age 13. Her blood pressure was 110/75 mm Hg, and her heart rate was 87 bpm. The physical examination was remarkable for mild jugular venous distension and pericardial knock. Liver function tests, electrolyte levels, cardiac enzymes, coagulation parameters, and urinalysis were within normal limits. Her ECG showed normal sinus rhythm, rightward axis, and intraventricular conduction delay. Chest x-ray revealed mediastinal enlargement with mild cardiomegaly, pericardial calcification, and right pleural effusion. Two-dimensional echocardiography demonstrated enhanced echogenicity of the pericardium suggesting the presence of calcification, biatrial enlargement, normal left ventricular dimensions, and systolic function (ejection fraction 59%), and abnormal ventricular septal motion. Cardiovascular magnetic resonance imaging confirmed pericardial thickening with calcification, presence of constrictive physiology with septal bounce, and abrupt cessation of left ventricle diastolic filling. Cardiac catheterization revealed equalization of end-diastolic pressures, and interdependence of ventricular pressures consistent with constrictive physiology. Coronary angiogram demonstrated the obliteration of the second obtuse marginal branch of left circumflex artery in systole and diastole, and compression of the first obtuse marginal branch that induced mid to late diastolic interruption of coronary flow (Video). The patient was referred to surgery and pericardiectomy was performed. Histopathologic examination of the pericardium excluded mycobacterium tuberculosis or granulomatous diseases. The patient was discharged home following surgery and her symptoms were improved during the postoperative follow-up.
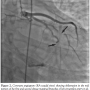 Discussion. We describe here a case of constrictive pericarditis associated with compression of the first and second obtuse marginal branch of circumflex coronary artery. Compression of coronary arteries with pericardial constriction is an unusual condition. Our case represents an unusual presentation with angina pectoris and signs and symptoms of myocardial ischemia along with right heart failure. Angina can be related to a calcification originating from the pericardium and constraining the coronary artery, or due to diastolic filling of the left ventricle that compresses the vessel against the fixed noncompliant pericardium.
Discussion. We describe here a case of constrictive pericarditis associated with compression of the first and second obtuse marginal branch of circumflex coronary artery. Compression of coronary arteries with pericardial constriction is an unusual condition. Our case represents an unusual presentation with angina pectoris and signs and symptoms of myocardial ischemia along with right heart failure. Angina can be related to a calcification originating from the pericardium and constraining the coronary artery, or due to diastolic filling of the left ventricle that compresses the vessel against the fixed noncompliant pericardium.
Goldberg et al described a patient with tuberculous pericarditis who had complete obliteration of an obtuse marginal branch of the left circumflex artery, due to pericardial calcification infiltrating the coronary artery.11 Ours and Goldberg’s case underline the importance of the timing of the compression of the coronary vessel during the cardiac cycle. Diastolic stenosis of coronary arteries is suggestive of external compression rather than arterial obliteration. On the other hand, diastolic compression of coronary artery is not a specific sign of constrictive pericarditis, since it has also been reported in patients with increased diastolic ventricular pressure.5,6 The coronary flow pattern can distinguish the underlying etiology of coronary compression. If the compression begins early in diastole, this finding suggests increased intraventricular pressures. On the other hand, if the compression of the coronary artery occurs in mid to late diastole, it suggests decreased compliance of the overlying pericardium. In the case presented here, the relief of diastolic compression after pericardiectomy and the presence of prevalent compression of coronary artery during mid-late diastole were suggestive of pericardial constriction. We conclude that angiographic demonstration of native coronary artery compression in the presence of right heart failure symptoms can suggest pericardial constriction, and the compression of the coronary artery in mid to late diastole might be a specific sign of pericardial constriction. Once a diagnosis is made, extensive resection of the pericardium rather than partial decortication limited to the anterior and lateral surface of the ventricles is mandatory to avoid recurrence of pericardial constriction following surgery. Several reports in the literature12,13 support this strategy of treatment as they revealed recurrent pericardial constriction following partial pericardiectomy with residual pericardium in the posterior surface of the heart.
In conclusion, patients with constrictive pericarditis can develop angina pectoris secondary to pericardial constraint on the native coronary arteries. These patients can be treated with pericardiectomy. Constrictive pericarditis should be kept in mind in patients who present with angina pectoris and right heart failure.
References
- Sadikot RT, Fredi JL, Light RW. A 43-year-old man with a large recurrent right-sided pleural effusion. Diagnosis: Constrictive pericarditis. Chest. 2000;117(4):1191-1194.
- Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100(13):1380-1386.
- Kalaria VG, Koradia N, Breall JA. Myocardial Bridge: a clinical review. Catheter Cardiovasc Interv. 2002;57(4):552-556.
- Angelini P, Trivellato M, Donis J, Leachman RD. Myocardial bridges: a review. Prog Cardiovasc Dis. 1983;26(1):75-88.
- Angelini P, Leachman RD, Autrey A. Atypical phasic coronary artery narrowing. Cathet Cardiovasc Diagn. 1986;12(1):39-43.
- Hongo M, Goto T, Watanabe N, et al. Relation of phasic coronary flow velocity profile to clinical and hemodynamic characteristics of patients with aortic valve disease. Circulation. 1993;88(3):953-960.
- Yoshikawa J, Akasaka T, Yoshida K, Takagi T. Systolic coronary flow reversal and abnormal diastolic flow patterns in patients with aortic stenosis: assessment with an intracoronary Doppler catheter. J Am Soc Echocardiogr. 1993;6(5):16-24.
- Akasaka T, Yoshikawa J, Yoshida K, Maeda K, Takagi T, Miyake S. Phasic coronary flow characteristics in patients with hypertrophic cardiomyopathy: a study by coronary Doppler catheter. J Am Soc Echocardiogr. 1994;7(1):9-19.
- Garg RK, Anderson AS, Jolly N. Diastolic coronary artery compression in a cardiac transplant recipient: treatment with a stent. Catheter Cardiovasc Interv. 2005;65(2):271-275.
- Chokshi SK, Meyers SN. Diastolic segmental compression of saphenous vein bypass graft. Am Heart J. 1989;118(2):402-404.
- Goldberg E, Stein J, Berger M, Berdoff RL. Diastolic segmental coronary artery obliteration in constrictive pericarditis. Cathet Cardiovasc Diagn. 1981;7(2):197-202.
- Tirilomis T, Unverdorben S, von der Emde J. Pericardectomy for chronic constrictive pericarditis: risks and outcome. Eur J Cardiothorac Surg. 1994;8(9):487-492.
- Nataf P, Cacoub P, Dorent R, et al. Results of subtotal pericardiectomy for constrictive pericarditis. Eur J Cardiothorac Surg. 1993;7(5):252-255; discussion 255-256.
__________________________________________
From the 1Texas Heart Institute and St. Luke's Episcopal Hospital, Houston, TX; the 2Institute of Cardiology, “G. d’Annunzio” University, Chieti, Italy.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted October 21, 2011 and accepted December 6, 2011.
Address for correspondence: Maher Nasser, MD, Texas Heart Institute and St. Luke, 6624 Fannin Street, Suite 1710, Houston, Texas 77030. Email: mnassermd@yahoo.com












