Role of Hybrid Endovascular Suite in Improving Outcomes of Surgical Ligation of Coronary Artery Fistula
ABSTRACT: Coronary artery fistulae are rare congenital or acquired coronary artery anomalies that can lead to significant cardiovascular morbidity and mortality. Surgical ligation has long been utilized in the treatment of these abnormalities. However, there is a high rate of recurrence due to incomplete closure of the fistulae, especially when multiple channels are present. Transcatheter techniques have become an acceptable alternative with good outcomes. Nevertheless, not all fistulae are amenable to the transcatheter approach and surgical repair is the treatment of choice. Intraoperative coronary angiography can improve the outcomes of surgery but has only sparingly been used due to the technical difficulties in a standard operating suite. Hybrid suites are becoming quite common these days with the emergence of procedures such as endovascular stent grafting and percutaneous valves. These suites have a complete imaging set up like a traditional catheterization laboratory and are also full operating suites. This case report discusses the use and potential benefits of performing intraoperative coronary angiography in a dedicated hybrid suite to help guide and ensure complete surgical closure of all fistulous connections.
J INVASIVE CARDIOL 2012;24(1):E10-E12
___________________________________________
Coronary artery fistulae (CAF) are abnormal communications between coronary arteries and any cardiac chamber or great vessels. They have an incidence of 0.1%-0.2% during adult coronary angiography.1-4 Most fistulae are discovered incidentally and are small and asymptomatic.5 However larger fistulae can lead to significant cardiovascular morbidity and mortality, mostly by way of inducing cardiac ischemia due to shunting of blood away from the coronary circulation.1 Surgical ligation has been the conventional gold standard for treatment of symptomatic CAF. With advancements in transcatheter techniques, this modality has become an acceptable alternative to surgery.6 However, some fistulae may not be suitable for transcatheter closure. Surgical ligation is still the best option for these patients. Unfortunately, there is a variable and often relatively high rate of recurrence.
Case Report. A 17-year-old female athlete presented with a 6-year history of worsening palpitations, dyspnea, and syncope. She also complained of chest pain with exertion.
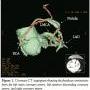 An event monitor was completed and showed sinus arrhythmia and intermittent junctional rhythm, but no other significant arrhythmias. An electrophysiologist also evaluated her and thought that her syncope was neurocardiogenic in nature. Because of exertional chest discomfort, an exercise echocardiogram was performed. She exercised 12 minutes and 47 seconds with no symptoms or electrocardiographic abnormalities. The left ventricular function was normal. However, it did show striking anteroseptal hypokinesis with exercise. This prompted evaluation with a coronary computed tomography angiogram (CCTA), which confirmed the presence of multiple coronary artery-to-pulmonary artery fistulae, originating from the left main, left anterior descending (LAD), and right coronary arteries (RCA) (Figure 1).
An event monitor was completed and showed sinus arrhythmia and intermittent junctional rhythm, but no other significant arrhythmias. An electrophysiologist also evaluated her and thought that her syncope was neurocardiogenic in nature. Because of exertional chest discomfort, an exercise echocardiogram was performed. She exercised 12 minutes and 47 seconds with no symptoms or electrocardiographic abnormalities. The left ventricular function was normal. However, it did show striking anteroseptal hypokinesis with exercise. This prompted evaluation with a coronary computed tomography angiogram (CCTA), which confirmed the presence of multiple coronary artery-to-pulmonary artery fistulae, originating from the left main, left anterior descending (LAD), and right coronary arteries (RCA) (Figure 1).
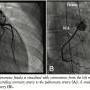 Due to the presence of exercise-induced ischemia that was thought to be from coronary “steal” phenomenon, the patient underwent left heart catheterization with planned coiling of the fistulae. Coronary angiography demonstrated the presence of multiple CAF originating from multiple sites in the left main, proximal LAD, and also the proximal RCA (Figures 2A and B). Due to the very tortuous nature of the channels and difficult angle of origin, coil embolization was unsuccessful due to inability to successfully cannulate these channels.
Due to the presence of exercise-induced ischemia that was thought to be from coronary “steal” phenomenon, the patient underwent left heart catheterization with planned coiling of the fistulae. Coronary angiography demonstrated the presence of multiple CAF originating from multiple sites in the left main, proximal LAD, and also the proximal RCA (Figures 2A and B). Due to the very tortuous nature of the channels and difficult angle of origin, coil embolization was unsuccessful due to inability to successfully cannulate these channels. 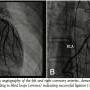 After discussion with the family, interventional cardiology, and the cardiothoracic surgery team, we proceeded with surgical ligation. Due to the numerous fistulous communications from multiple arteries, there was concern that all channels may not be ligated, and this may result in residual shunt. In order to facilitate complete closure of the CAF, we performed the procedure in a state-of-the-art hybrid cardiovascular suite with complete availability of a ceiling mounted flat panel imaging system and also fully furnished cardiac operating room. Intraoperative TEE confirmed the angiography findings and showed brisk fistula flow into the main pulmonary artery. Sternotomy was made and we performed surgical exploration and ligation. Intraoperative, post-ligation, coronary angiography confirmed complete closure of all the fistulous tracts with residue of fistulae that end blindly (Figures 3A and B).
After discussion with the family, interventional cardiology, and the cardiothoracic surgery team, we proceeded with surgical ligation. Due to the numerous fistulous communications from multiple arteries, there was concern that all channels may not be ligated, and this may result in residual shunt. In order to facilitate complete closure of the CAF, we performed the procedure in a state-of-the-art hybrid cardiovascular suite with complete availability of a ceiling mounted flat panel imaging system and also fully furnished cardiac operating room. Intraoperative TEE confirmed the angiography findings and showed brisk fistula flow into the main pulmonary artery. Sternotomy was made and we performed surgical exploration and ligation. Intraoperative, post-ligation, coronary angiography confirmed complete closure of all the fistulous tracts with residue of fistulae that end blindly (Figures 3A and B).
Discussion. CAF are defined as abnormal communication between any coronary artery and any of the cardiac chambers or great vessels.7 They are uncommon and most are congenital defects that arise as a result of incomplete embryonic development. They can also be acquired as a result of collagen vascular disease, chest trauma, or cardiac surgery. CAF most commonly involve the RCA but can involve both coronary arteries in about 5% of patients.8 Most patients remain asymptomatic for several years, but eventually develop symptoms, including fatigue, dyspnea, angina, and/or heart failure. The angina is usually exertional, due to shunting of blood from the coronary artery to the pulmonary circulation with exercise, leading to ischemia.9
Small fistulae can remain asymptomatic and usually will spontaneously close. More sizable fistulae get progressively larger over time and are prone to symptoms and complications, such as thrombosis, ischemia, arrhythmia, endocarditis, or rupture.10-13 Therefore, patients with large fistulae associated with symptoms or ischemia should undergo percutaneous or surgical closure due to the tendency of fistulae to dilate over time, increasing the severity of symptoms and the risk of complications.14
Traditionally surgical ligation has been performed for these patients.15 With the evolution of endovascular techniques, transcatheter embolization techniques have become an acceptable alternative to surgery for patients with coronary artery fistulae. Multiple different techniques have been used successfully including coils,16,17 detachable balloons,18,19 umbrellas,20 polyvinyl alcohol foam,21 and other occlusive devices.22 Although these methods have been successful, the use of implantable coils is considered the best method due to the low risk and improved delivery techniques. The transcatheter approach, if performed by an experienced operator, is usually safe and procedural complications are rare. These complications include transient ischemic changes, device embolization, myocardial infarction, and arrhythmias.6 The transcatheter approach may be preferred over the surgical approach due to lower cost, shorter recovery time, and less morbidity, including avoidance of thoracotomy or sternotomy and cardiopulmonary bypass.23
However, some fistulae are not suitable for the transcatheter approach due to multiple connections, tortuous routes, and acute angles making catheter positioning difficult.6 Surgical ligation is the best option for these patients. The traditional technique involves a median sternotomy and epicardial dissection and ligation with or without cardiopulmonary bypass. If anatomic considerations are not favorable for the epicardial approach, an open heart operation with cavitary exposure is employed.24,25 Reported success rates are high, though recurrence rates of up to 20% have been reported in literature.2,26 These are mostly due to incomplete ligation or missed fistulous tracts, especially in those with numerous fistulae. Intraoperative coronary angiography can be used to help ensure complete closure of all fistulous communications, helping to eliminate further procedures for recurrence. Hol et al described their experience with 7 patients over a 12-year period. Four patients were treated with the aid of on the table angiography, while 3 were not. Successful and complete CAF closure was obtained in all 4 angiography-guided cases while 2 of the 3 others had residual fistulae on follow-up imaging.24 While these authors confirmed the value of intraoperative angiography, it is generally not used routinely due to the inconvenience of bringing a mobile coronary angiography unit to the operating suite and also due to less optimal image quality.
Over the last few years however, there has been a steady proliferation of hybrid suites especially designed for smooth performance of several procedures including endovascular abdominal and thoracic aortic aneurysm repair; peripheral and carotid artery stenting; repair of congenital anomalies; hybrid coronary revascularization utilizing stenting with off-pump coronary artery bypass grafting; and percutaneous valve replacement or repair, to name a few.27,28 By doing these procedures in a minimally invasive way, patients can avoid the inherent morbidity of cardiopulmonary bypass and associated prolonged intensive care stays. Additionally, complex cases are more easily treated since the suite is designed to handle minimally invasive hybrid operations and open surgical procedures.27
When developing a hybrid suite, careful planning and professional expertise is important. These hybrid suites must be large enough to house additional imaging and anesthesia equipment and combine surgical sterility with flat-panel imaging and workstations.27 The suites are well equipped to perform endovascular procedures with the ability to quickly convert to an open procedure if needed. They also allow for immediate pre-procedure imaging to provide the most accurate anatomical information and post-procedure imaging to ensure quality control.29
Fully integrated hybrid endovascular suites come at a significant cost. They require specialized equipment including an imaging system, which allows for higher tube heat capacity and measurement capabilities requiring higher resolution and superior image quality. A carbon fiber surgical table is needed to optimize the usefulness of the radiographic equipment. The costs could rise higher with the addition of even more sophisticated imaging modalities. Additionally, most states require lead-lined walls for operating rooms with fixed imaging systems, which can be quite costly. However, this integrated setting saves time and money by allowing more procedures to be completed in the same room by the existing staff without needing to relocate equipment. Furthermore, the suite becomes a one stop shop where patients can get diagnosed and treated in one visit, leading to quicker recoveries and shorter hospital stays.27
In our case, a hybrid room was ideally suited for surgical repair of the coronary fistulae as it allowed for angiography to be readily available and provided improved image quality. We suggest that this hybrid technology should be routinely used for all such cases to optimize outcomes.24
References
- Vavuranakis M, Bush CA, Boudoulas H. Coronary artery fistulas in adults: incidence, angiographic characteristics, natural history. Cathet Cardiovasc Diagn. 1995 Jun;35(2):116-120.
- Cheung DL, Au WK, Cheung HH, Chiu CS, Lee WT. Coronary artery fistulas: long-term results of surgical correction. Ann Thorac Surg. 2001 Jan;71(1):190-195.
- Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990 Sep;21(1):28-40.
- Gillebert C, Van Hoof R, Van de Werf F, Piessens J, De Geest H. Coronary artery fistulas in an adult population. Eur Heart J. 1986 May;7(5):437-443.
- Latson LA. Coronary artery fistulas: how to manage them. Catheter Cardiovasc Interv. 2007 Jul 1;70(1):110-116.
- Armsby LR, Keane JF, Sherwood MC, Forbess JM, Perry SB, Lock JE. Management of coronary artery fistulae. Patient selection and results of transcatheter closure. J Am Coll Cardiol. 2002 Mar 20;39(6):1026-1032.
- Levin DC, Fellows KE, Abrams HL. Hemodynamically significant primary anomalies of the coronary arteries. Angiographic aspects. Circulation. 1978 Jul;58(1):25-34.
- Baim DS, Kline H, Silverman JF. Bilateral coronary artery--pulmonary artery fistulas. Report of five cases and review of the literature. Circulation. 1982 Apr;65(4):810-815.
- Schamroth C. Coronary artery fistula. J Am Coll Cardiol. 2009 Feb 10;53(6):523.
- McNamara JJ, Gross RE. Congenital coronary artery fistula. Surgery. 1969 Jan;65(1):59-69.
- Habermann JH, Howard ML, Johnson ES. Rupture of the coronary sinus with hemopericardium. A rare complication of coronary arteriovenous fistula. Circulation. 1963 Dec;28:1143-1144.
- Moro-Serrano C, Martinez J, Madrid AH, et al. Ventricular tachycardia in a patient with congenital coronary arteriovenous fistula. Am Heart J. 1992 Aug;124(2):503-505.
- Corvaja N, Moses JW, Vogel FE, et al. Exercise-induced ventricular tachycardia associated with coronary arteriovenous fistula and correction by transcatheter coil embolization. Catheter Cardiovasc Interv. 1999 Apr;46(4):470-472.
- Chee TS, Tan PJ, Koh SK, Jayaram L. Coronary artery fistula diagnosed by transthoracic Doppler echocardiography. Singapore Med J. 2007 Oct;48(10):e262-264.
- Kamiya H, Yasuda T, Nagamine H, et al. Surgical treatment of congenital coronary artery fistulas: 27 years' experience and a review of the literature. J Card Surg. 2002 Mar-Apr;17(2):173-177.
- Reidy JF, Anjos RT, Qureshi SA, Baker EJ, Tynan MJ. Transcatheter embolization in the treatment of coronary artery fistulas. J Am Coll Cardiol. 1991 Jul;18(1):187-192.
- Issenberg HJ. Transcatheter coil closure of a congenital coronary arterial fistula. Am Heart J. 1990 Dec;120(6 Pt 1):1441-1443.
- Hartnell GG, Jordan SC. Balloon embolisation of a coronary arterial fistula. Int J Cardiol. 1990 Dec;29(3):381-383.
- Skimming JW, Gessner IH, Victorica BE, Mickle JP. Percutaneous transcatheter occlusion of coronary artery fistulas using detachable balloons. Pediatr Cardiol. 1995 Jan-Feb;16(1):38-41.
- Perry SB, Rome J, Keane JF, Baim DS, Lock JE. Transcatheter closure of coronary artery fistulas. J Am Coll Cardiol. 1992 Jul;20(1):205-209.
- Strunk BL, Hieshima GB, Shafton EP. Treatment of congenital coronary arteriovenous malformations with micro-particle embolization. Cathet Cardiovasc Diagn. 1991 Feb;22(2):133-136.
- McMahon CJ, Nihill MR, Kovalchin JP, Mullins CE, Grifka RG. Coronary artery fistula. Management and intermediate-term outcome after transcatheter coil occlusion. Tex Heart Inst J. 2001;28(1):21-25.
- Zhu XY, Zhang DZ, Han XM, et al. Transcatheter closure of congenital coronary artery fistulae: immediate and long-term follow-up results. Clin Cardiol. 2009 Sep;32(9):506-512.
- Hol PK, Geiran O, Andersen K, et al. Improvement of coronary artery fistula surgery by intraoperative imaging. Ann Thorac Surg. 2004 Dec;78(6):2193-2195.
- Mavroudis C, Backer CL, Rocchini AP, Muster AJ, Gevitz M. Coronary artery fistulas in infants and children: a surgical review and discussion of coil embolization. Ann Thorac Surg. 1997 May;63(5):1235-1242.
- Rittenhouse EA, Doty DB, Ehrenhaft JL. Congenital coronary artery- cardiac chamber fistula. Review of operative management. Ann Thorac Surg. 1975 Oct;20(4):468-485.
- Kpodonu J, Raney A. The cardiovascular hybrid room a key component for hybrid interventions and image guided surgery in the emerging specialty of cardiovascular hybrid surgery. Interact Cardiovasc Thorac Surg. 2009 Oct;9(4):688-692.
- Kpodonu J. Hybrid cardiovascular suite: the operating room of the future. J Card Surg. 2010 Nov;25(6):704-709.
- Sikkink CJ, Reijnen MM, Zeebregts CJ. The creation of the optimal dedicated endovascular suite. Eur J Vasc Endovasc Surg. 2008 Feb;35(2):198-204.
___________________________________________
From the 1Division of Cardiovascular Diseases, University of Kansas Medical Center, 2Mid America Thoracic and Cardiovascular Surgeons, and 3Mid America Cardiology, Kansas City, Kansas.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted May 24, 2011, provisional acceptance given June 27, 2011, final version accepted July 27, 2011.
Address for correspondence: Kamal Gupta, MD, University of Kansas Medical Center, 3901 Rainbow Blvd, Kansas City, KS 66160. Email: kgupta@mac.md












