Relationship of Walking Impairment and Ankle-Brachial Index Assessments with Peripheral Arterial Translesional Pressure Gradients
Abstract: Background. The relationship of peripheral arterial mean translesional pressure gradient (TLG) to presenting symptom, functional impairment, and initial noninvasive ABI assessments has never been established. Objectives. To evaluate the association between TLG, severity of walking impairment, rest and exercise ankle-brachial indices (ABI). Methods. TLG in 19 patients presenting with claudication and single superficial femoral artery lesion were measured invasively. TLG was measured at rest and post-hyperemia induction with intra-arterial adenosine (100 and 200 µg), nitroglycerin (100 and 200 µg), and after 3 minutes of ipsilateral calf cuff pressure inflation-deflation sequence. For each patient, a walking impairment questionnaire (WIQ) was completed and rest and exercise ABI were measured prior to TLG assessment. Results. Mean age was 60 ± 6 years, 89% were men. Mean WIQ score was 4817 ± 3549, mean rest and exercise ABI were 0.79 ± 0.14 and 0.59 ± 0.17, respectively, and mean exercise duration was 6.3 ± 3.4 minutes. TLG with 100 µg of adenosine strongly correlates with WIQ score (r = -0.723); rest ABI (r = -0.748); exercise ABI (r = -0.888), exercise duration (r = -0.711), and percent angiographic stenosis (r = -0.818), respectively (p < 0.01 for all). TLG with adenosine 200 µg, nitroglycerin 100 and 200 µg and after cuff inflation-deflation also demonstrated significant correlation. Receiver operator curve analysis demonstrated that a TLG ≥ 11 mmHg post 100 µg adenosine administration had 71.43% sensitivity and 100% specificity for identifying patients with disease defining state of exercise ABI ≤ 0.70. Conclusion. This study validates the utility of invasive TLG measurements using vasodilation for determining the functional and hemodynamic significance of superficial femoral artery lesions.
J INVASIVE CARDIOL 2011;23:352–356
Key words: claudication, peripheral vascular disease, pressure gradient
__________________________________
Peripheral arterial disease (PAD) affects more than 30 million Americans and is a leading cause of cardiovascular morbidity, mortality, and socio-economic burden due to functional impairments and limb amputations.1,2 Though there have been significant strides in the diagnosis and treatment of PAD, there currently are no studies that provide invasive hemodynamic correlates of PAD symptoms and traditional screening tests such as the ankle-brachial index (ABI). Establishing these correlates along with discriminating thresholds for PAD disease state may help clinicians not only in the invasive assessment of patients with claudication or pseudoclaudication, but also in the evaluation of angiographically intermediate lesions, selection of endovascular treatment targets, along with success and durability of therapy.
To the best of our knowledge, this study is the first to report correlation of invasive infra-inguinal peripheral arterial mean translesional mean pressure gradients (TLG) with subjective [walking impairment questionnaire (WIQ)] and objective (rest and exercise ABI and exercise duration) measures of lower extremity claudication.
Methods
 Patients and non-invasive assessment. Fifty-two consecutive patients were screened and referred for the management of claudicaiton in this study. Of these, 33 patients with total vessel occlusions, multiple lesions, < 1-vessel infrapopliteal run-off, and/or presence of > 50% supra-inguinal lesions were excluded. Nineteen consecutive patients with lower extremity claudication who were found to have a single superficial femoral artery (SFA) lesion and at least 1-vessel infrapopliteal runoff were included. Initial evaluation of all patients consisted of physical examination, walking impairment assessment using a standardized WIQ3 (Appendix 1), rest ABI and treadmill exercise ABI using the Naughton protocol.4
Patients and non-invasive assessment. Fifty-two consecutive patients were screened and referred for the management of claudicaiton in this study. Of these, 33 patients with total vessel occlusions, multiple lesions, < 1-vessel infrapopliteal run-off, and/or presence of > 50% supra-inguinal lesions were excluded. Nineteen consecutive patients with lower extremity claudication who were found to have a single superficial femoral artery (SFA) lesion and at least 1-vessel infrapopliteal runoff were included. Initial evaluation of all patients consisted of physical examination, walking impairment assessment using a standardized WIQ3 (Appendix 1), rest ABI and treadmill exercise ABI using the Naughton protocol.4
The study was approved by the Institutional Review Board and all patients provided written informed consent.
Invasive assessment. All patients underwent peripheral angiography using digital subtraction. Quantitative assessment of lesion severity and length was performed using the Pie Medical Imaging computer-based automatic quantitative angiographic edge-detection system, version 5.4. The projection that best showed the stenosis in its tightest view was selected. The intra- and inter-observer correlation coefficients of the quantitative measurements ranged from 0.93–0.97 and 0.94–0.99, respectively.
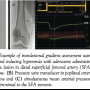 TLG measurements were performed by placing a straight tip end- hole catheter in the SFA proximal to the lesion. A 0.014˝ Certus pressure wire was advanced to the tip of the end-hole catheter and pressures were equalized. The pressure wire was advanced distal to the SFA stenosis and the resting TLG was recorded. Hyperemic TLG was subsequently measured after administration of 100 and 200 µg of adenosine, and 100 and 200 µg of nitroglycerin through the end-hole catheter (Figure 1). Before administration of each vasodilator type and dose, all pressure tracings were allowed to return to baseline. Ipsilateral lower extremity cuff-induced hyperemia was then induced by inflating a sphygmomanometer cuff 20–30 mmHg above the systolic pressure for 3 minutes followed by its rapid release and continuous pressure recordings as described above. After all recordings were completed, the pressure wire transducer was withdrawn to the tip of the delivery catheter and equalization of pressures was reconfirmed. In the rare event of observed drift of the pressure tracings, the entire analysis was redone with a new pressure wire. Systolic blood pressure and heart rate for all patient observations were maintained above 100 mmHg and 60 beats/minute, respectively. The operator was blinded to all recordings and was instructed by the recording team to perform each analysis step. This was achieved by turning the pressure wire display console away from the operator’s line of vision. The operator was able to monitor electrocardiographic tracing, arterial pressure tracing from the sheath, and oxygen saturation.
TLG measurements were performed by placing a straight tip end- hole catheter in the SFA proximal to the lesion. A 0.014˝ Certus pressure wire was advanced to the tip of the end-hole catheter and pressures were equalized. The pressure wire was advanced distal to the SFA stenosis and the resting TLG was recorded. Hyperemic TLG was subsequently measured after administration of 100 and 200 µg of adenosine, and 100 and 200 µg of nitroglycerin through the end-hole catheter (Figure 1). Before administration of each vasodilator type and dose, all pressure tracings were allowed to return to baseline. Ipsilateral lower extremity cuff-induced hyperemia was then induced by inflating a sphygmomanometer cuff 20–30 mmHg above the systolic pressure for 3 minutes followed by its rapid release and continuous pressure recordings as described above. After all recordings were completed, the pressure wire transducer was withdrawn to the tip of the delivery catheter and equalization of pressures was reconfirmed. In the rare event of observed drift of the pressure tracings, the entire analysis was redone with a new pressure wire. Systolic blood pressure and heart rate for all patient observations were maintained above 100 mmHg and 60 beats/minute, respectively. The operator was blinded to all recordings and was instructed by the recording team to perform each analysis step. This was achieved by turning the pressure wire display console away from the operator’s line of vision. The operator was able to monitor electrocardiographic tracing, arterial pressure tracing from the sheath, and oxygen saturation.
After all peak TLG assessments were recorded, the data and pressure tracings were downloaded to a dedicated analysis workstation for off-line analysis. Fractional flow reserves (FFR) were also measured, defined as the lowest ratio of the mean distal pressure to the mean proximal pressure during hyperemia. The decision to perform or defer endovascular treatment of the target SFA lesion was made blinded to the recorded pressure gradients at the discretion of the operator.
 Statistical analyses. Continuous variables were summarized as means ± 1 standard deviation and discrete variables were presented as frequencies and group percentages. The association between rest and hyperemia TLG with the WIQ score, rest ABI, exercise ABI, and maximal treadmill exercise time was assessed with the Spearman’s rho. Receiver operating characteristic (ROC) curves were used to determine discriminating thresholds of TLG for clinically significant patient functional impairment that was defined as an exercise ABI ≤ 0.70.5
Statistical analyses. Continuous variables were summarized as means ± 1 standard deviation and discrete variables were presented as frequencies and group percentages. The association between rest and hyperemia TLG with the WIQ score, rest ABI, exercise ABI, and maximal treadmill exercise time was assessed with the Spearman’s rho. Receiver operating characteristic (ROC) curves were used to determine discriminating thresholds of TLG for clinically significant patient functional impairment that was defined as an exercise ABI ≤ 0.70.5
Results
 Clinical characteristics and non-invasive assessment. The baseline characteristics of the study patients are shown in Table 1. Mean age was 60 ± 6 years and 89% were men, with a high prevalence of coronary artery disease risk factors (69%) and diabetes (42%). All patients presented with life-style limiting claudication. Mean WIQ score was 4817 ± 3549 (range: 0 to 14080, with 0 being unable to walk indoors performing activities of daily living and 14080 being the score of a person who can walk ≥ 1500 feet without limiting symptoms), mean rest and exercise ABI were 0.79 ± 0.14 and 0.59 ± 0.17, respectively, and mean exercise duration was 6.3 ± 3.4 minutes. Three patients had angiographic severity of SFA diameter stenosis < 25%. No patient had angiographically normal SFA. A statistically significant association was observed between ABI (both rest and exercise), exercise duration, and WIQ score (Figure 2).
Clinical characteristics and non-invasive assessment. The baseline characteristics of the study patients are shown in Table 1. Mean age was 60 ± 6 years and 89% were men, with a high prevalence of coronary artery disease risk factors (69%) and diabetes (42%). All patients presented with life-style limiting claudication. Mean WIQ score was 4817 ± 3549 (range: 0 to 14080, with 0 being unable to walk indoors performing activities of daily living and 14080 being the score of a person who can walk ≥ 1500 feet without limiting symptoms), mean rest and exercise ABI were 0.79 ± 0.14 and 0.59 ± 0.17, respectively, and mean exercise duration was 6.3 ± 3.4 minutes. Three patients had angiographic severity of SFA diameter stenosis < 25%. No patient had angiographically normal SFA. A statistically significant association was observed between ABI (both rest and exercise), exercise duration, and WIQ score (Figure 2).
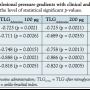 Invasive assessment. Mean TLG at baseline resting was 5.1 mmHg. Mean TLG following hyperemic challenge with intra-arterial administration of 100 µg of adenosine, 100 µg of nitroglycerin, 200 µg of nitroglycerin, and cuff inflation-deflation sequence were 12.7 mmHg, 14.2 mmHg, 14.8 mmHg, and 14.4 mmHg, respectively. Mean TLG with all hyperemic challenges were significantly greater than baseline resting translesional gradients (p < 0.01 for all). All TLG measurements had high correlation with WIQ score, rest and exercise ABI, exercise duration, and percent angiographic lesion stenosis (Table 2). The Spearman’s correlation coefficient between TLG and exercise ABI was consistently > 0.850. There was also a strong correlation between TLG and FFR (Figure 3).
Invasive assessment. Mean TLG at baseline resting was 5.1 mmHg. Mean TLG following hyperemic challenge with intra-arterial administration of 100 µg of adenosine, 100 µg of nitroglycerin, 200 µg of nitroglycerin, and cuff inflation-deflation sequence were 12.7 mmHg, 14.2 mmHg, 14.8 mmHg, and 14.4 mmHg, respectively. Mean TLG with all hyperemic challenges were significantly greater than baseline resting translesional gradients (p < 0.01 for all). All TLG measurements had high correlation with WIQ score, rest and exercise ABI, exercise duration, and percent angiographic lesion stenosis (Table 2). The Spearman’s correlation coefficient between TLG and exercise ABI was consistently > 0.850. There was also a strong correlation between TLG and FFR (Figure 3).
 Table 3 demonstrates ROC curve analysis for identifying patients with exercise ABI ≤ 0.70. Fourteen out of the 19 patients had exercise ABI ≤ 0.7. The TLG after 100 µg of adenosine administration had the greatest area under the curve. A cut-off of 11 mmHg had 71.43% sensitivity and 100% specificity for detecting patients with exercise ABI ≤ 0.70.
Table 3 demonstrates ROC curve analysis for identifying patients with exercise ABI ≤ 0.70. Fourteen out of the 19 patients had exercise ABI ≤ 0.7. The TLG after 100 µg of adenosine administration had the greatest area under the curve. A cut-off of 11 mmHg had 71.43% sensitivity and 100% specificity for detecting patients with exercise ABI ≤ 0.70.
Discussion
 The main finding of our study is that invasive measurement of TLG is feasible for SFA lesions and has a strong correlation with subjective (WIQ scores) and objective measures (rest and exercise ABI) of lower extremity claudication. To date, this is the first study to report this association in patients with symptomatic PAD. A threshold TLG ≥ 11 mmHg post 100 µg adenosine administration is best at identifying patients with symptomatic PAD.
The main finding of our study is that invasive measurement of TLG is feasible for SFA lesions and has a strong correlation with subjective (WIQ scores) and objective measures (rest and exercise ABI) of lower extremity claudication. To date, this is the first study to report this association in patients with symptomatic PAD. A threshold TLG ≥ 11 mmHg post 100 µg adenosine administration is best at identifying patients with symptomatic PAD.
It also demonstrates that similar to coronary invasive pressure measurements, adenosine may be used to induce hyperemia for adequate evaluation of infra-inguinal arterial stenoses, although the other hyperemic challenges that were evaluated also provided similar results.
Invasive hyperemic pressure measurements have been extensively studied in the coronary circulation.6 Coronary FFR has been validated as an accurate measure of flow-limiting stenosis leading to myocardial ischemia and is currently being used to determine whether a coronary lesion might derive benefit from revascularization. Although TLG was the primary measure in this study, these reported correlations are consistent with FFR as well.
In the peripheral circulation, hyperemic invasive pressure measurements to evaluate lesions have only been reported in the renal artery.7 Our study finds an association between an invasive hemodynamic measure (TLG) and non-invasive measures (WIQ, ABI) in the infra-inguinal arterial distribution. In addition, we found that TLG ≥ 11 mmHg after 100 µg of intra-arterial adenosine was 100% specific and sensitive (71.43%), thereby providing the greatest discriminating ability to diagnose symptomatic PAD. Lower extremity hyperemic TLG could have several important applications in daily clinical practice. It could allow determination of the functional severity of arterial lesions in patients who undergo angiography for PAD symptoms, potentially target treatment of such lesions, and assess response to endovascular treatment.
The limitations of this study include a small sample size with single, focal SFA lesions without significant lower extremity arterial inflow and infra-popliteal outflow disease. Although all the vasodilators evaluated were effective in inducing hyperemia to detect stenosis, the vasodilators and dosages used were selected solely based on what has been reported in literature and based upon clinical experience of the investigators.8,9
Conclusion
Invasive assessment of TLG provides an excellent correlate of PAD symptoms and ABI assessments. These data can be useful in guiding endovascular strategies in the treatment of flow-limiting stenosis in the SFA.
Listen to a podcast with Dr. Banerjee based upon this research at https://invasivecardiology.com/content/walking-impairment-and-abi.
References
- Hampering JL, Foster V. Meeting the challenge of peripheral arterial disease. Arch Intern Med. 2003;163(8):877-878.
- Belch JJF, Topol EJ, Agnelli G, et al. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med. 2003;163(8):884-892.
- Nicolaï S, Kruidenier LM, Rouwet EV, et al. The walking impairment questionnaire: an effective tool to assess the effect of treatment in patients with intermittent claudication. J Vasc Surg. 2009;50(1):89-94.
- Naughton J, Balke B, Nagle F. Refinements in method of evaluation and physical conditioning before and after myocardial infarction. Am J Cardiol. 1964;14:837-843.
- Vogt MT, Wolfson SK, Kuller LH. Lower extremity arterial disease and the aging process: a review. J Clin Epidemiol. 1991;45(5):529-542.
- Pijls NHJ, De Bruyne B, Peels K, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334(26):1703-1708.
- De Bruyne B, Manoharan G, Pijls NH, et al. Assessment of renal artery stenosis severity by pressure gradient measurements. J Am Coll Cardiol. 2006;48(9):1851-1851. Epub 2006 Oct 17.
- Garcia LA, Carrozza JP. Physiologic evaluation of translesion pressure gradients in peripheral arteries: comparison of pressure wire and catheter-derived measurements. J Interv Cardiol. 2007;20(1):63-65.
- Bragadeesh T, Ibrahim S, Pascotto M, et al. Detection of peripheral vascular stenosis by assessing skeletal muscle flow reserve. J Am Coll Cardiol. 2005;45(5):780-785.
__________________________________
From 1the VA North Texas Health Care System and 2the University of Texas Southwestern Medical Center, Dallas, Texas.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Banerjee reports speaker honoraria from St. Jude Medical, Medtronic, and Johnson & Johnson and research support from Boston Scientific and The Medicines Company. Dr. Brilakis reports speaker honoraria from St Jude Medical and Terumo; research support from Abbott Vascular and InfraRedx; and salary from Medtronic (spouse). No other authors report conflicts regarding the content herein. Manuscript submitted March 21, 2011, provisional acceptance given April 21, 2011, final version accepted June 30, 2011.
Address for correspondence: Subhash Banerjee, MD, 4500 S. Lancaster Road (111a), Dallas, TX 75216. Email: subhash.banerjee@utsouthwestern.edu














