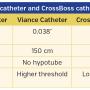
Recanalization of Popliteal and Infrapopliteal Chronic Total Occlusions Using Viance and CrossBoss Crossing Catheters: A Multicenter Experience From the XLPAD Registry
Abstract: Background. Chronic total occlusions (CTOs) are reported in up to 40% of patients with symptomatic peripheral arterial disease. The Viance Crossing catheter (Covidien) and the CrossBoss catheter (Boston Scientific) are novel devices that facilitate either true lumen or subintimal navigation across CTOs. The purpose of this study was to examine the acute procedural success of these devices for popliteal and below-the-knee (BTK) CTOs. Methods. Clinical and procedural outcome data between Sept 2010 and October 2013 were analyzed from the ongoing multicenter XLPAD registry. Technical success was defined as true lumen passage of the catheter, while procedural success was defined as successful vessel revascularization inclusive of subintimal passage and reentry. Results. Twenty-nine patients underwent 31 procedures, in which 37 lesions were treated with the Viance and CrossBoss catheters. Mean age of the group was 69.1 ± 10.7 years and 26 patients were male (90%). All patients (100%) had hypertension, 62% were diabetic, and 48% were active smokers. Critical limb ischemia was the indication for the procedure in 58% of cases; mean Rutherford class was 4.2 ± 1.2 for the entire cohort. Target CTOs included 14 anterior tibial, 9 posterior tibial, 5 peroneal, 1 tibio-peroneal trunk, and 8 popliteal artery lesions. Mean lesion length was 81 ± 64 mm; 15 lesions (41%) were severely calcified. True lumen passage was obtained in 24 lesions (65% technical success). Subintimal entry was achieved in 5 lesions (14%). Procedural success was achieved in 26 lesions (70%). Procedural failure was related to longer lesion length (P<.001), and mean length of failed lesions was 136 ± 65 mm. Conclusion. Viance and CrossBoss catheters were associated with an overall 70% procedural success with predominantly true lumen crossing in a BTK-CTO cohort. CTO length remains an important determinant of device success.
J INVASIVE CARDIOL 2015;27(1):2-7
Key words: chronic total occlusion, Viance crossing catheter, peripheral arterial disease
_______________________________________________________
Due in large part to the rising prevalence of diabetes and peripheral arterial disease (PAD) in the United States, critical limb ischemia (CLI) is a growing epidemic. Approximately 800,000 non-traumatic amputations are performed annually in the US, with substantial morbidity and mortality.1 Endovascular therapy is a key component in the management of CLI patients. With advances in equipment and techniques, non-surgical therapies are often the first choice for treatment of below-the-knee (BTK) atherosclerotic disease.
The anatomical challenges associated with BTK disease treatment include the frequent presence of vascular calcification, a high prevalence of chronic total occlusions (CTOs), and diffuse atherosclerosis. CTOs in particular are present in as many as 40% of patients presenting with CLI. The presence of a CTO has been associated with a lower procedural success rate with endovascular therapy, as well as increased procedural times, radiation dose, and contrast load.2 Failure to cross the occlusion is the primary method of failure in the majority of unsuccessful cases.2 The use of a wire coupled with a support catheter has been documented to cross infrainguinal CTOs in 40%-60% of cases.3 With the advent of subintimal passage and reentry with hydrophilic guidewires, the success rates have increased to over 80% (although this statistic is largely limited to above-the-knee occlusions).4,5 Success rates for infrapopliteal vessels are lower, with treatment failure occurring in about 25% of lesions <15 cm long and a failure rate of ~46% for occlusions ≥15 cm long.6 Subintimal tracking and reentry remain challenging in BTK arteries due to smaller vessel size. These factors are the rationale for developing novel peripheral CTO crossing catheters. We present a multicenter experience with the Viance (Covidien) and CrossBoss (Boston Scientific) crossing catheters — novel CTO crossing devices when used for popliteal and BTK lesions in patients with either severe claudication or CLI. Our aim was to define the variables that are associated with acute procedural success for CTO revascularization in this context.
Methods
Clinical and procedural data. Clinical and procedural outcome data on Viance and CrossBoss catheter use between Sept 2010 and October 2013 were analyzed from the ongoing multicenter XLPAD registry.7 The purpose of the XLPAD registry is to assess outcomes in patients undergoing endovascular therapy of infrainguinal peripheral arterial disease. The target number of patients for enrollment is approximately 2174. Currently, data from 600 patients and 900 procedures have been recorded. There are currently 10 sites across the US participating in the study. The University of Texas Southwestern Medical Center at Dallas supports the registry. All data are entered online into the REDCAP data entry portal maintained by the University of Texas Southwestern at Dallas. A total of 486 CTO procedures have been entered into the registry.8

As a part of data entry into REDCAP, chart review was performed to collect demographic, clinical, and procedural information on popliteal and BTK peripheral CTO lesions when the Viance or CrossBoss catheter was used either as an initial crossing strategy or following failed guidewire and crossing catheter technique. Procedural information was collected after reviewing the peripheral angiograms and procedural notes by an experienced individual blinded to the clinical details. CTO length, degree of calcification, and choice of anticoagulation, access site, and sheath size were noted. CTO length was defined as the distance between the proximal and distal caps on angiography using a radiopaque ruler. Intraprocedural complications (flow-limiting dissections, perforations, distal embolization) and acute postprocedural complications (bleeding) were recorded. Data on the use and success of reentry devices either with the Outback catheter (Cordis Corporation) or Enteer Reentry System (Covidien) were noted. The Enteer is a balloon-assisted reentry catheter device.
A single CTO was defined based on the presence of a single occlusion or multiple sequential occlusions separated by ≤2 cm in the popliteal arteries and a single occlusion or sequential occlusions separated by ≤1 cm with a patent distal runoff in the BTK vessels. Vascular calcification was classified as none, mild, moderate, or severe based on the presence of either an isolated focus of calcification (mild), contiguous segments of calcification on one or alternating sides of the vessel (moderate), or contiguous calcification on both sides of the vessel (severe) as visible on angiographic views prior to contrast injection. The presence of collaterals at the proximal and distal ends of the CTO was assessed in a semiquantitative method (none, poorly developed, or well developed).
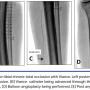
Outcome definitions. Two different study outcomes were defined: technical success and procedural success. Technical success was defined as recanalization and placement of a guidewire beyond the distal CTO cap into the true lumen of the target vessel with the use of Viance and CrossBoss alone. Distal true lumen entry was confirmed by angiographic contrast injection. Since intravascular ultrasound (IVUS) was not routinely used, it is possible that the catheter went subintimal and reentered the lumen; however, technical success was contingent upon traversing the CTO without the use of adjunctive reentry devices. When the Viance or CrossBoss tracked subintimally and the reentry device was needed, the case was deemed a technical failure in relation to use of the crossing catheter. In these procedures, when a reentry device was used to achieve distal true lumen reentry, the final case was reported as a procedural success.
Statistical analysis. Continuous parameters are reported as mean ± standard deviation, and discrete variables are reported as numbers and percentages. Continuous variables were compared using Student’s t-test and non-parametric analyses were performed using Spearman rank order correlation. Multivariable logistic regression models were used to determine independent predictors of procedural and technical success. A P-value of <.05 was considered statistically significant. Given the small overall sample size, both the univariable and multivariable analyses were considered exploratory and hypothesis generating. All statistics were performed using SigmaStat/Plot version 11.2.0.5 (Systat).
 Results
Results
Twenty-nine patients were identified who underwent 31 endovascular procedures for 37 CTO lesions with the use of the Viance (n = 28) or CrossBoss catheter (n = 9). Two patients had separate CTO revascularization procedures for both limbs. Four patients had 2 CTO lesions attempted and 1 patient had 3 CTO lesions attempted on the same index procedure. Baseline demographic data are reported in Table 1. Procedural data are shown in Table 2. The majority of patients were male (90%), with a mean age of 69.1 ± 10.7 years. All procedures were performed on patients with severe claudication or CLI with mean Rutherford class of 4.2 ± 1.2. Eleven patients (38%) had evidence of tissue loss.
 Contralateral access via common femoral artery was used in 27 procedures (87%) and ipsilateral antegrade access of the common femoral artery was used in 5 cases (13%). Fourteen lesions (38%) were noted to be in the anterior tibial artery, 9 (24%) in the posterior tibial artery, 5 (14%) in the peroneal artery distribution, 1 (3%) in the tibioperitoneal trunk, and the remaining 8 (22%) in popliteal artery. Only 1 lesion had undergone a prior crossing attempt at a separate setting with a support catheter and guidewire technique; the remaining interventions were de novo attempts. Fifteen lesions (41%) were severely calcified, 1 lesion had moderate calcification, and 4 lesions (11%) had mild calcification. Average CTO length was 81 ± 64 mm (range, 15-250 mm). Most of the proximal caps and distal caps were well defined (95% and 84%, respectively). Twenty-one lesions (57%) had extensive collateral development. Bivalirudin was used for anticoagulation in 11 procedures (35%) and unfractionated heparin in the other 20 cases (65%). Multiple 0.014˝ guidewires were used inside the Viance/CrossBoss for the various cases, including hydrophilic-coated, heavy-tipped CTO wires, supportive body wires, and “workhorse” wires, with no correlation between wire type and technical success (data not shown).
Contralateral access via common femoral artery was used in 27 procedures (87%) and ipsilateral antegrade access of the common femoral artery was used in 5 cases (13%). Fourteen lesions (38%) were noted to be in the anterior tibial artery, 9 (24%) in the posterior tibial artery, 5 (14%) in the peroneal artery distribution, 1 (3%) in the tibioperitoneal trunk, and the remaining 8 (22%) in popliteal artery. Only 1 lesion had undergone a prior crossing attempt at a separate setting with a support catheter and guidewire technique; the remaining interventions were de novo attempts. Fifteen lesions (41%) were severely calcified, 1 lesion had moderate calcification, and 4 lesions (11%) had mild calcification. Average CTO length was 81 ± 64 mm (range, 15-250 mm). Most of the proximal caps and distal caps were well defined (95% and 84%, respectively). Twenty-one lesions (57%) had extensive collateral development. Bivalirudin was used for anticoagulation in 11 procedures (35%) and unfractionated heparin in the other 20 cases (65%). Multiple 0.014˝ guidewires were used inside the Viance/CrossBoss for the various cases, including hydrophilic-coated, heavy-tipped CTO wires, supportive body wires, and “workhorse” wires, with no correlation between wire type and technical success (data not shown).
 The Viance/CrossBoss catheters were successful in true lumen passage in 24 lesions, resulting in a 65% technical success rate. Subintimal entry occurred in 5 lesions (14%). The Enteer system was used in 3 of these lesions and was unsuccessful in achieving reentry in the distal true lumen. The Outback catheter was used for 1 other lesion, and achieved successful reentry. A support catheter along with multiple wire attempts was used for subintimal tracking and reentry in 1 lesion after the Enteer had failed, resulting in successful distal true lumen access. Thus, procedural success was achieved in 26 lesions (70%). Twenty-six lesions were noted to be <100 mm; the Viance was able to traverse 23 of these via true lumen and 1 lesion subintimally, accounting for 88% technical and 92% procedural success. When several lesions were attempted on the same procedure, oftentimes the attempt to cross the second CTO was curtailed to prevent further contrast load and radiation exposure, decreasing the overall success rates. However, 25 patients (80%) had at least 1 infrapopliteal CTO that was successfully recanalized, resulting in establishment of at least 1 in-line vessel to the foot.
The Viance/CrossBoss catheters were successful in true lumen passage in 24 lesions, resulting in a 65% technical success rate. Subintimal entry occurred in 5 lesions (14%). The Enteer system was used in 3 of these lesions and was unsuccessful in achieving reentry in the distal true lumen. The Outback catheter was used for 1 other lesion, and achieved successful reentry. A support catheter along with multiple wire attempts was used for subintimal tracking and reentry in 1 lesion after the Enteer had failed, resulting in successful distal true lumen access. Thus, procedural success was achieved in 26 lesions (70%). Twenty-six lesions were noted to be <100 mm; the Viance was able to traverse 23 of these via true lumen and 1 lesion subintimally, accounting for 88% technical and 92% procedural success. When several lesions were attempted on the same procedure, oftentimes the attempt to cross the second CTO was curtailed to prevent further contrast load and radiation exposure, decreasing the overall success rates. However, 25 patients (80%) had at least 1 infrapopliteal CTO that was successfully recanalized, resulting in establishment of at least 1 in-line vessel to the foot.
Atherectomy was performed in 11 lesions (30%) and distal embolization protection with a filter device was used with atherectomy in 7 lesions (19%). Balloon angioplasty was performed in all treated lesions. After the procedure, stenosis of the treated lesions was 10.0 ± 10%. Stents were used after balloon angioplasty in only 2 lesions. Contrast usage was 189 ± 72 mL, with 28 ± 16 minutes of fluoroscopy time. The total average procedure time recorded was 116 ± 51 minutes. There was no correlation between the lesion length with the fluoroscopy time and total procedure time. There was 1 intraprocedural complication (development of thrombus within the posterior tibial artery managed with eptifibatide infusion without subsequent clinical sequelae).
Univariable correlates with technical success included shorter lesion length (r = -0.559; P=.01), and a well-defined distal CTO cap (r = 0.537; P<.001). Correlates with procedural success included shorter lesion length (r = -0.675; P<.001), a well-defined distal cap (r = 0.608; P<.001), and successful true lumen passage of the Viance catheter (r = 0.884; P<.001). Procedural success was also associated with lower Rutherford score (r = -0.339; P=.04). Lesion calcification was not related to technical success (r = 0.145; P=.39) or procedural success (r = 0.158; P=.35), but was related to longer procedural times (r = 0.548; P=.01). No demographic variable, including the presence of diabetes, age, chronic kidney disease, or current smoking, was related to technical or procedural success. In a multivariable logistic model that included lesion characteristics, lesion length was the only independent predictor of technical success (odds ratio, 0.984; 95% confidence interval, 0.969-1.00; P=.04) or procedural success (odds ratio, 0.977; 95% confidence interval, 0.959-0.995; P=.01).
Discussion
The Viance and CrossBoss catheters were developed by Bridgepoint Medical, Inc. Both catheters employ a similar technique for CTO recanalization. This involves a fast manual torque maneuver with minimal forward pressure to either probe and penetrate the CTO cap/lumen or alternatively enter into the subintimal space in a controlled fashion. The CrossBoss catheter is designed for coronary CTO recanalization and the Viance catheter is indicated for peripheral CTO therapy. The differences between these two catheters are highlighted in Table 4. The Viance catheter was acquired by Covidien in January 2012 and was later approved by the US Food and Drug administration for peripheral vascular use.9 The Viance catheter was first available commercially in September 2012. Prior to the availability of the Viance catheter, the CrossBoss was used off-label in the present study. Boston Scientific has subsequently acquired the CrossBoss catheter for coronary use.
In the present study, Viance and CrossBoss catheters were able to achieve 65% technical success and 70% procedural success rates in recanalizing popliteal and infrapopliteal CTOs. These data are also consistent with the finding that infrapopliteal CTO procedural outcomes tend to be poorer than those for femoropopliteal CTOs. However, when the initial target lesion was only included in the present analysis, the procedural success rate was 80% — suggesting that the efficacy of this device may be higher for selected lesions. To date, the largest evaluation of the Viance catheter has been the Peripheral Facilitated Antegrade Steering Technique in Chronic Total Occlusions (PFAST-CTOs) study.10 In this study, a total of 66 patients underwent CTO recanalization with the Viance catheter with or without the Enteer system. The majority of patients had Rutherford 3-4 claudication, and the target lesion was the superficial femoral artery in 65.2%. The catheter was used in 13.6% of the popliteal vessels and in 16.7% of the tibial vessels. The perforation rate was 1.5% (1 case). The technical success rate in crossing the CTO was 84% (45 cases) with the Viance alone, and 86% (21 cases) with the assistance of the Enteer device. Approximately 42% of all lesions were reported to be moderate or severely calcified. Our present series represents the largest evaluation of the Viance device for popliteal and infrapopliteal disease. Our lower success rates likely reflect the challenges associated with BTK endovascular therapy — particularly therapy in smaller, diffusely diseased vessels.
Role of crossing device technology. Failure to cross into the true distal lumen is the most common reason for procedural failure in the context of CTO revascularization.2,11 CTO recanalization has traditionally been achieved with the use of a support catheter technique and wire (often with a weighted and/or hydrophilic tip) with either negotiation of the true lumen or subintimal passage and reentry.12 Given the challenges associated with reentry in the smaller BTK vessels and risk of damage to run-off vessels, dedicated crossing technologies are being developed to maintain true lumen position during recanalization. In theory, devices that facilitate true lumen crossing have several advantages over traditional methods, including reductions in procedural and radiation exposure times, as well as reduced risk of vessel damage. These devices may also result in a more attractive substrate for atherectomy. These potential benefits remain to be tested and are uncertain, largely due to a lack of randomized controlled data.
Several of the available tools to facilitate intraluminal crossing for both above-the-knee and BTK-CTOs are summarized in Table 3. The Frontrunner XP catheter (Cordis Endovascular) employs blunt microdissection to displace plaque.13 The Crosser catheter (Bard Peripheral Vascular) uses high-frequency vibrations propagated by a stainless-steel tip to facilitate penetration of hard and calcified lesions.14 The Wildcat catheter (Avinger) has a rotatable tip equipped with distal spiral wedges that can be used to bore a track into the CTO cap that facilitates guidewire placement distally.15 The Ocelot catheter (Avinger) uses a similar mechanism as the Wildcat catheter; however, it uses real-time optical coherence tomography for direct visualization to direct intravascular orientation.16,17 The Safe-Cross system (IntraLuminal Therapeutics) combines an optical coherence reflectometer with radiofrequency energy delivered from the wire tip to help the operator remain intraluminal.18 The TruePath CTO device19,20 (Boston Scientific) features a rotating diamond-coated tip designed to break through plaque in peripheral lesions. As noted in Table 3, the majority of studies examining the utility of dedicated crossing devices have focused on above-the-knee lesions (primarily in the superficial femoral artery). It should be noted that to date there are few peer-reviewed published papers available for dedicated BTK crossing technology data.
Viance catheter for infrapopliteal CTOs: design, tips, and techniques. In contrast to the devices described above, the Viance catheter is an over-the-wire 0.014˝-guidewire compatible, 150 cm-long, multiwire coiled shaft that has a 0.037˝ atraumatic distal tip. It has a hydrophilic stainless-steel distal coating and is compatible through a 5 Fr sheath. It is designed to deliver a guidewire via true lumen or subintimal pathways.
The catheter is advanced to the proximal CTO cap and manually spun using the torqueable handle. The torque device should be placed close to the entry of the catheter into the sheath with approximately <1 cm of separation. Spinning of the torque device should be performed in a rapid fashion in a single direction; this “fast-spin” technique allows the device to “find” microchannels through the occlusion. Upon finding a channel, the device tip will move forward — often relatively suddenly. The temptation to apply forward pressure should be avoided while the device tip probes the cap/channel, as this may increase subintimal passage. In our experience, up to 5 minutes of spinning may be required prior to visible forward movement of the device. If torque transmission is hindered, then an audible clicking will occur; in this case, spinning of the device in the opposite direction may be required. If failure of torque transmission does occur, it is an important troubleshooting step to ensure that the catheter is straight on the field and not looped or coiled, and that there is no wrapping of sterile towels or drapes along the shaft of the device. The Viance catheter shaft/tip is minimally shapeable, making altering its direction in a vessel challenging. If the catheter is migrating into an unwanted direction, spinning in the opposite direction may bring it into a different plane/direction. Although there are no published data, the wire characteristics/choice used with the Viance catheter may impact its performance. In our experience, we have noted the following wire techniques in combination with the Viance: (1) placing a curve on the tip of the guidewire and leading with the curved wire may help redirect the device in the desired direction; (2) leading with a heavy-tipped CTO wire may allow the device to initially engage the cap of the CTO; (3) the use of a stiff shaft wire may help maintain a straight orientation of the catheter; and (4) the distance of the wire from the distal end of the Viance appears to impact the freedom of movement of the Viance tip — such that maintaining the guidewire 1-2 cm proximal from the tip allows for greater probing movement of the Viance.
Study limitations. There are numerous study limitations to consider. Since the data were collected as part of a registry, the patients were non-randomized. Therefore, the role of selection bias cannot be underestimated as the decision to use the Viance or CrossBoss catheter on a given case was left up to the individual operators. It is unclear whether the successful cases might have been also successful with alternative crossing tools. Also, a comparison of the impact of the different construction of these two devices (Viance vs CrossBoss) in regard to efficacy is unclear because of the small number of cases. Calcification is classically associated with poor success rates in crossing CTOs. Calcification was not significantly related to treatment failure in the present study. Assessment of calcification severity can be difficult in the infrapopliteal circulation, as delineating intraluminal calcium from medial calcification can be challenging. Of note, calcification was associated with longer overall procedural times. No intravascular imaging modality was used to confirm true luminal crossing of the entire CTO, and only angiographic contrast injection into the distal vessel was used as confirmation for analysis. Also, the cost effectiveness of the Viance catheter in comparison to the standard catheter and wire technique needs to be examined in a larger study. In addition, the assessment of adjunctive tools for subintimal to true lumen reentry (particularly the role of the Enteer device) cannot be deciphered from the present study given the small number of cases.
Conclusion
In the present series, the Viance and CrossBoss crossing catheters appeared to be safe devices with acceptable efficacy in crossing popliteal and infrapopliteal occlusions. Lesion length appears to be the strongest predictor failure. Further larger registry and randomized data will be needed to better understand the role of this device in peripheral CTO therapy.
References
- Bell D. Peripheral arterial disease overview. Podiatry Management. 2009:209-216.
- Javed U, Laird JR. Specialty crossing devices: understanding the learning curve. Endovasc Today. 2012:52-57.
- Bolia A, Miles KA, Brennan J, et al. Percutaneous transluminal angioplasty of occlusions of the femoral and popliteal arteries by subintimal dissection. Cardiovasc Interv Radiol. 1990;13(6):357-363.
- Markose G, Miller FN, Bolia A. Subintimal angioplasty for femoro-popliteal occlusive disease. J Vasc Surg. 2010;52(5):1410-1416.
- London NJ, Srinivasan R, Naylor AR, et al. Subintimal angioplasty of femoropopliteal artery occlusions: the long-term results. Eur J Vasc Surg. 1994;8(2):148-155.
- Sato T. Current situation of below the knee intervention in the patients of CLI (critical limb ischemia). J Vasc Interv Radiol. 2011;22(3):S39.
- Banerjee S. Multi-center registry for peripheral arterial disease interventions and outcomes (XLPAD). ClinicalTrials.gov identifier: NCT01904851. July 2013.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) — a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed inform. 2009;42(2):377-381. Epub 2008 Sep 30.
- Javed U, Laird JR. Specialty crossing devices: understanding the learning curve. Endovasc Today. 2012;5:52-57.
- Gray W. Peripheral facilitated antegrade steering technique in chronic total occlusions (pFAST - CTO). Presented at the Vascular Interventional Advances (VIVA) Conference, Las Vegas, Nevada: 2012.
- Mossop PJ, Amukotuwa SA, Whitbourn RJ. Controlled blunt microdissection for percutaneous recanalization of lower limb arterial chronic total occlusions: a single center experience. Catheter Cardiovasc Interv. 2006;68(2):304-310.
- Adams GL, Gardner SJ, Gardner J, et al. Exotic access, techniques, and devices for infrapopliteal CTOs. Endovasc Today. 2012;5:44-49.
- Shetty R, Vivek G, Thakkar A, et al. Safety and efficacy of the Frontrunner XP catheter for recanalization of chronic total occlusion of the femoropopliteal arteries. J Invasive Cardiol. 2013;25(7):344-347.
- Gandini R, Volpi T, Pipitone V, et al. Intraluminal recanalization of long infrainguinal chronic total occlusions using the Crosser system. J Endovasc Ther. 2009;16(1):23-27.
- Pigott JP, Raja ML, Davis T, et al. A multicenter experience evaluating chronic total occlusion crossing with the Wildcat catheter (the CONNECT study). J Vasc Surg. 2012;56(6):1615-1621. Epub 2012 Sep 11.
- Selmon M. CONNECT II study. Presented at the Vascular Interventional Advances (VIVA) Conference, Las Vegas, Nevada: 2012.
- Cawich I, Marmagkiolis K, Cilingiroglu M. Ocelot catheter for the treatment of long SFA occlusion. Catheter Cardiovasc Interv. 2014;83(1):144-147. Epub 2013 Oct 7.
- Kirvaitis RJ, Parr L, Kelly LM, et al. Recanalization of chronic total peripheral arterial occlusions using optical coherent reflectometry with guided radiofrequency energy: a single center experience. Catheter Cardiovasc Interv. 2007;69(4):532-540.
- TruePath CTO Device ReOpen study data. https://www.bostonscientific.com/truepath/clinical-data.html. Boston Scientific, 2013.
- George JC. Revascularization of calcified infrapopliteal chronic total occlusion. Vascular Disease Management. 2013;10:56-58.
- Joye J. The PATRIOT (Peripheral Approach To Recanalization In Occluded Totals) study results. Transcatheter Cardiovascular Therapeutics, Oral abstract #42; 2007.
____________________________________________________________________
From the 1Department of Cardiology, University of Texas Health Science Center, San Antonio, Texas; 2Department of Cardiology, University of Texas Southwestern Medical Center, Dallas, Texas; and 3Longview Regional Medical Center, Longview, Texas.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Prasad is a member of the speaker’s bureau for Aztra Zeneca; institution received funds related to training courses on Covidien products. Dr Banerjee reports grants from Boston Scientific and The Medicines Company; personal fees from Gilead, St. Jude, Cordis Corporation, Boehringer Ingerheim, Sanofi, and Medtronic; ownership of MdcareGlobal; and intellectual property with HygeiaTel. The remaining authors report no conflicts to disclose.
Manuscript submitted March 27, 2014, provisional acceptance given June 6, 2014, final version accepted July 28, 2014.
Address for correspondence: Anand Prasad, MD, FACC, FSCAI, UT Health Science Center at San Antonio, Department of Medicine, Division of Cardiology, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. Email: anandprasadmd@gmail.com