Primary Percutaneous Coronary Intervention in a Case of Idiopathic Spontaneous Coronary Artery Dissection
ABSTRACT: Spontaneous coronary artery dissection (SCAD) is a rare but important cause of acute coronary syndrome. SCAD can cause unstable angina, acute myocardial infarction, and sudden death. The diagnosis of coronary dissection is usually made by coronary angiography. Therapeutic options include medical therapy, percutaneous coronary intervention, and bypass surgery. A 23-year-old male patient presented with acute inferior wall myocardial infarction. He was taken for primary percutaneous coronary intervention and found to have long segment SCAD in the right coronary artery. He was stented with 2 drug-eluting stents; TIMI 3 flow was restored; and the patient had an uneventful, complete recovery.
J INVASIVE CARDIOL 2012;24(4):E84-E86
Key words: Acute myocardial infarction, coronary artery dissection, drug-eluting stents
_____________________________________________
Spontaneous coronary artery dissection (SCAD) can occur at random or as a consequence of chest trauma, cardiac surgery, coronary angiography, coronary intervention, or as an extension of aortic dissection. Dissection of the coronary artery results in separation of the layers of the arterial wall, creating a false lumen. The separation may be between the intima and the media, or between the media and the adventitia. Hemorrhage into the false lumen can impinge upon the true lumen of the coronary artery, impairing blood flow and causing myocardial ischemia, infarction, or sudden death.
SCAD has traditionally been described in 3 groups of patients: 1) patients with coronary artery disease, 2) women during the peripartum period, and 3) a heterogeneous group of patients with idiopathic disease, comprising individuals without cardiovascular risk factors, those with connective tissue disorders (Marfan syndrome, Ehlers-Danlos syndrome, etc.), and cases of intoxication.
Case Report
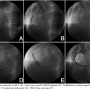 A 23-year-old man was admitted with an acute onset of severe chest pain at rest. The pain was retrosternal, associated with shortness of breath, and diaphoresis. His past medical history was unremarkable. He is a non-smoker and non-diabetic. He had no history of drug abuse. His blood pressure was 110/76 mm Hg, with a pulse of 110 BPM.
A 23-year-old man was admitted with an acute onset of severe chest pain at rest. The pain was retrosternal, associated with shortness of breath, and diaphoresis. His past medical history was unremarkable. He is a non-smoker and non-diabetic. He had no history of drug abuse. His blood pressure was 110/76 mm Hg, with a pulse of 110 BPM.
Cardiac examination was unremarkable for murmurs, rubs, or gallops. S4 was present. There were no manifestations of connective tissue diseases, with negative assays for lupus anticoagulant, anticardiolipin antibodies (IgG and IgM), and antinuclear antibodies. Lipid parameters, lipoprotein a, and homocysteine levels were within normal limits. Hematological, renal, and other biochemical parameters were also normal.
His electrocardiogram revealed ST elevation in leads II, III, and avf. He was treated with intravenous nitrates, aspirin, clopidogrel, beta-blocker, and atorvastatin. After receiving eptifibatide, he was transferred for coronary angiography and subsequent intervention. Cardiac catheterization revealed a long dissection in the right coronary artery (RCA) from mid segment to the distal part with thrombus. The other coronary arteries were normal. The left ventricle had akinesia in the infero-posterior segments. The ejection fraction was 35%. RCA ostium was engaged with right Judkins 3.5 7 Fr guide catheter. The area of dissection was crossed with a BMW guidewire with difficulty. Aspiration catheter did not cross. A 2 mm x 10 mm balloon (Sprinter Legend, Medtronic) was placed into the distal portion of the RCA and inflated sequentially to prepare the bed. Two endeavor resolute stents (3.5 mm x 24 mm, 4.0 mm x 30 mm) were deployed at 16 atm across the lesion. Both the stents were post-dilated. TIMI III flow restored with resolution of the ST segment elevations, and abolishment of chest pain. Intracoronary adenosine and nicorandil were given beforehand.
His cardiac enzymes reached an early peak of 450 U/L (reference range 0-145 U/L), with CKMB > 130 ng/mL (reference range 0.0-4.9 ng/mL), and troponin T > 21 ng/mL (reference range 0.00-0.10 ng/mL). The patient was started on oral medications, which included aspirin, clopidogrel, ramipril, metoprolol, and atorvastatin. His hospital course was uneventful and he was discharged home on day 5. In subsequent follow-up during the past year the patient had no recurrent chest pain or other cardiac symptoms. Stress test is negative and ejection fraction is 60%.
Discussion
Epidemiology. Clinical presentation ranges from asymptomatic to unstable angina, acute myocardial infarction, ventricular arrhythmias, and sudden death. The overall incidence of SCAD in angiographic series ranges from 0.28% to 1.1%. 70% of SCAD occurs in women; of that, approximately 30% occurs in the peripartum period. In most reported cases the diagnosis was established at autopsy. Although not invariable, left coronary artery dissections are more common in women, while the RCA is usually involved in men. Overall, the left anterior descending artery is affected in 75% of cases, the RCA in 20% of cases, the left circumflex artery in about 4% of cases, and left main coronary artery in <1% of cases.1 The most common conditions associated with SCAD are coronary atherosclerosis and the peripartum period.
Pretty first described SCAD in 1931 at autopsy in a 42-year-old woman.2 DeMaio et al have described 83 cases; 62 were diagnosed at autopsy and 21 were found antemortem. A series of 21 patients with the antemortem diagnosis of SCAD is reported and follow-up is provided for 16 of the 21 previously reported cases. Of the 62 autopsy cases, 10 (16%) were men (mean age 47) and 52 (84%) were women (mean age 40). Dissection of the left anterior descending coronary artery predominated in this group of patients, occurring in 80% of men and 65% of women.3
Pathogenesis. The most common conditions associated with SCAD are coronary atherosclerosis and the peripartum period. The etiology of spontaneous coronary artery dissection remains unclear. Atherosclerotic plaque inflammation and rupture may cause disruption of the intimal-medial junction, resulting in an intimal flap and subsequent intramural hematoma formation. In contrast, peripartum dissection has been associated with the presence of eosinophils. Eosinophilic infiltrates have been described in the coronary artery adventitia in autopsy studies of SCAD without coronary atherosclerosis. Eosinophil granules contain numerous lytic substances, including collagenase, peroxidase, major basic protein, and acid phosphatase.4
Hemorrhage from weakened vasa vasorum in the outer tunica media leading to compression of the lumen and subsequent dissection has been proposed as another possible mechanism leading to SCAD.
Elevations of estrogen and progesterone occur during pregnancy and the peripartum period. In addition, during pregnancy there are microstructural changes in the elastic and collagen fibers of the tunica media of the aorta that may be caused by hormonal and hemodynamic factors. These changes could also occur in the coronary arteries, possibly contributing to the predisposition to dissection in the peripartum period.
Spasm may increase the shear stress on coronary arteries and lead to dissection. Variant angina has been associated with spontaneous coronary dissection. Cocaine causes coronary spasm; vasoconstriction and coronary dissection has been reported with cocaine use. Conditions that cause abnormalities in arterial wall structure like Marfan’s syndrome, Ehlers-Danlos syndrome, and lysyl oxidase deficiency have been associated with SCAD. Vasculitis such as polyarteritis nodosa, systemic lupus erythematosus, and isolated eosinophilic arteritis can involve the coronary arteries and cause dissection. There have been reports of spontaneous dissection in patients with antiphospholipid syndrome and inflammatory bowel disease. Medications such as oral contraceptives, cyclosporine, 5-fluorouracil, and fenfluramine have been associated with SCAD. Excessive exercise has been linked to dissection. The typical appearance of coronary artery dissection on coronary angiography is a thin radiolucent line representing the intimal medial flap with flow in 2 separate lumens. The caliber of the artery may be irregular and haziness may be present indicating thrombus formation, especially during acute coronary syndromes. Rarely, the artery appears narrowed without evidence of intimal flap. This can occur when there is no flow of contrast through the false lumen; for example, in dissections with an entry point but no exit, or in dissections limited to the medial-adventitial layers. In these cases, intravascular ultrasound can visualize the dissection flap. The false lumen is often filled with organizing thrombus, which appears heterogeneous and speckled.
Diagnosis and management. The diagnosis of SCAD is usually made by coronary angiography. There is increased recognition of SCAD due to advances in imaging modalities, including coronary angiography, intravascular ultrasound, and CT angiography. No guidelines currently exist for the management of SCAD owing to the condition’s scarcity. Treatment options for SCAD include medical therapy, percutaneous coronary intervention (PCI), or coronary artery bypass surgery. Dissection of large coronary arteries causing persistent ischemia is usually treated with percutaneous intervention or surgery, while dissections of small vessels are treated medically. Treatment of SCAD is similar to that of acute coronary syndromes. This includes anticoagulation with heparin, aspirin, clopidogrel, beta-blockers, and nitrates. Calcium channel blockers may be used to treat spasm. When cardiac catheterization is not available, SCAD patients presenting with acute ST elevation myocardial infarction have been treated with thrombolytics. Coronary obstruction in SCAD is not due to intraluminal thrombus; instead, an intimal flap with intramural hematoma compresses the lumen, and thrombolytics may promote extension of the dissection.
Koller et al reported the successful use of immunosuppressive therapy with prednisone and cytoxan in a 35-year-old postpartum woman with 5 noncontiguous dissections. Angiographic resolution was noted at 3 months. This suggests that inflammation and eosinophilic infiltration may have a predominant role in the etiology of SCAD.5 Glycoprotein IIb/IIIa inhibitors have been utilized to treat ischemia due to coronary dissection and are often given prior to coronary angiography for high-risk unstable angina and ST-elevation myocardial infarction before the diagnosis of coronary dissection is known. Glycoprotein IIb/IIIa inhibitors may be used as adjunctive therapy during percutaneous intervention for unstable dissections; however, there is a theoretical risk of hematoma expansion with these agents and more data are needed.
Simon Cheung et al described a case of SCAD in a 44-year-old woman with mitral valve prolapse. Coronary angiography showed coronary artery dissection in an LAD with 90%-95% obstruction and reduced (TIMI I) flow. The patient was treated with continued glycoprotein IIb/IIIa inhibitor (tirofiban) infusion. Angiographic resolution with return of prompt (TIMI III) flow was noted in their patient.6 Percutaneous intervention with stenting can restore flow in the true lumen, relieving ischemia, and seal the dissection, preventing further expansion. Intravascular ultrasound can aid in diagnosis, particularly when an angiographic intimal flap is not evident. Technical issues during PCI include placing the guidewire in the true lumen rather than the dissection plane and ensuring sealing of the dissection entry point with an appropriately sized stent. IVUS can be used to confirm guidewire placement in the true lumen, evaluate the length of dissection, vessel size, and assess stent apposition and sealing of the dissection. The clinical success rate of stenting in patients with SCAD is over 90%.7 Single vessel dissections are usually treated with percutaneous intervention with stenting while left main dissection, multivessel involvement, or failure of percutaneous interventional procedures may require surgical intervention. Hong et al initially described successful stenting in 1996. An important limitation is represented by the risk of coronary perforation in the case of entry and passage of the guidewire into the false lumen.
The prognosis of patients with SCAD has improved in recent years, likely due to increased diagnosis by cardiac catheterization and advances in treatment. In earlier series, mortality from spontaneous dissection was approximately 50%. If the patient survived the original episode, subsequent survival was 80%, and approximately 50% of patients experienced a recurrent dissection within 2 months. With contemporary medical therapy, the rate of recurrent dissection is much lower and most patients are asymptomatic at follow-up, with a 95% survival and 5% recurrent dissection rate.8
Conclusion
SCAD is a rare cause of acute coronary syndrome. It should always be suspected in young patients especially in young females during pregnancy. There is no consensus regarding the treatment of SCAD. Conservative medical therapy, PCI, and surgery have all been used successfully. Intracoronary stenting appears to be a valid treatment option in patients with SCAD with a high success rate. Drug-coated stents offer an additional advantage by reducing the risk of stent-related neointimal growth.
References
- Jorgensen MB, Aharonian V, Mansukhani P, Mahrer PR. Spontaneous coronary dissection: a cluster of cases with this rare finding. Am Heart J. 1994;127(5):1382-1387.
- Pretty HC. Dissecting aneurysm of coronary artery in a woman aged 42. Br Med J. 1931;1:667.
- DeMaio SJ Jr , Kinsella SH, Silverman ME. Clinical course and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 1989;64(8):471-474.
- Moukarbel GV, Alam SE. Spontaneous coronary artery dissections: management options in the stent era. J Invasive Cardiol. 2004;16(6):333-335.
- Koller PT, Cliffe CM, Ridley DJ. Immunosuppressive therapy for peripartum-type spontaneous coronary artery dissection: case report and review. Clinical Cardiol. 1998;21(1):40-46.
- Cheung S, Mithani V, Watson RM. Healing of spontaneous coronary dissection in the context of glycoprotein llB/lllA inhibitor therapy: a case report. Catheter Cardiovasc Interv. 2000;51(1):95-100.
- Kamran M, Guptan A, Bogal M. Spontaneous coronary artery dissections: case series and review. J Invasive Cardiol. 2008;20(10):553-559.
- Oka S, Manabe K, Gondo H, Hiramatsu Y, Kagiyama Y, Gotou I. Jpn Circ J. 1997;61(11):954-957.
_____________________________________________
From the Institute of Post Graduate Medical Education and Research, Calcutta, India.
Disclosure: The author has completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The author reports no conflicts of interest regarding the content herein.
Manuscript submitted September 15, 2011, provisional acceptance given October 11, 2011, final version accepted December 7, 2011.
Address for correspondence: Goutam Datta, MD, DNB, DM, Assistant Professor, Department of Cardiology, Institute of Post Graduate Medical Education and Research, P3 Lake Gardens, Calcutta—700033, India. E-mail: goutamdattadn@yahoo.in












