Performance of the Edwards Sapien 3 Ultra Transcatheter Aortic Valve System in Patients With Aortic Stenosis and Annulus Diameter in Proximity to Valve Size
Abstract
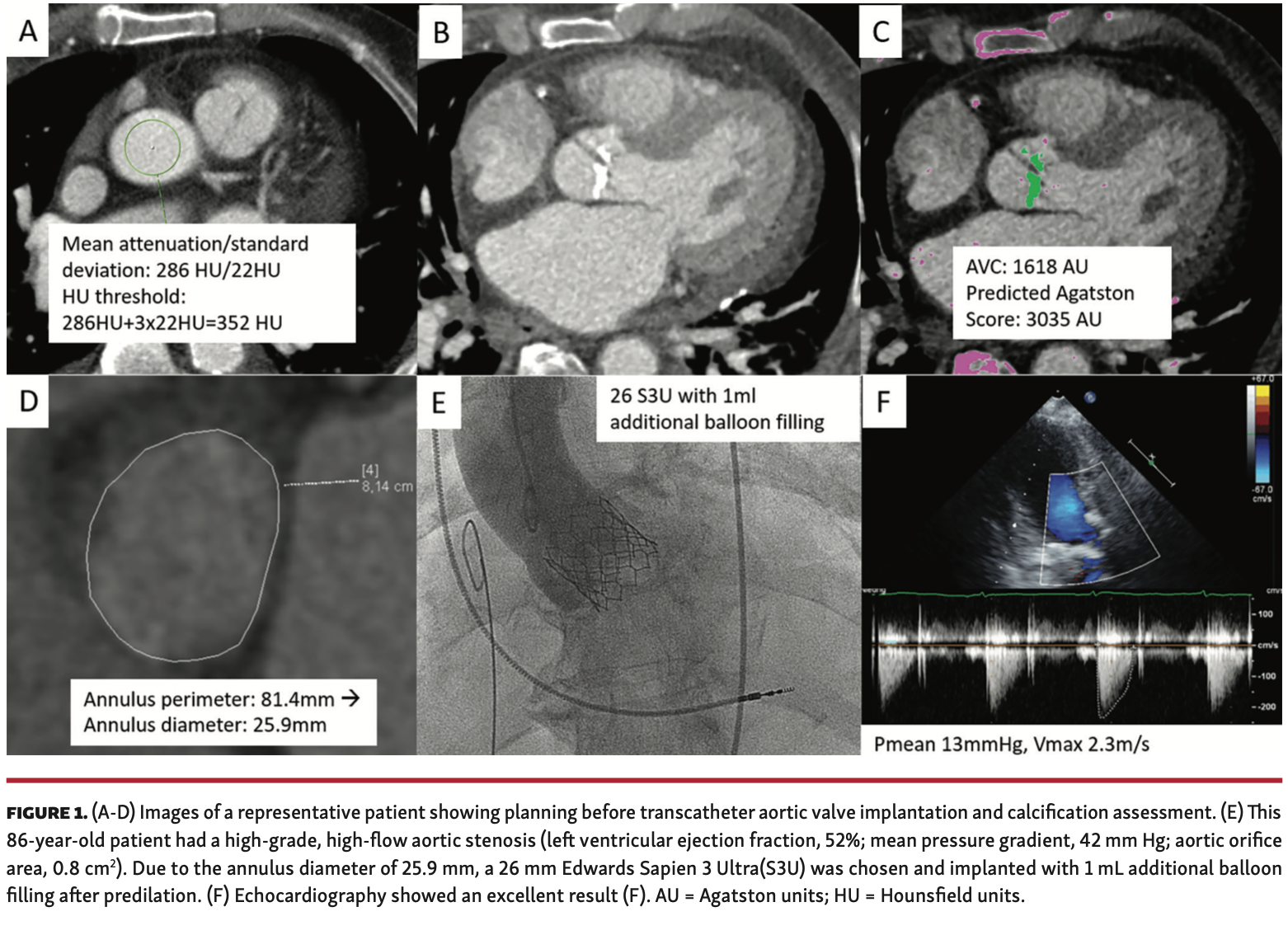
Objectives. The new Sapien 3 Ultra (S3U) transcatheter heart valve (Edwards Lifesciences) was designed with the intention to improve paravalvular sealing. In patients with an annulus size in proximity to the prosthesis size, little or no oversizing of the transcatheter aortic valve implantation (TAVI) prosthesis may lead to paravalvular regurgitation. Thus, this study was designed to assess valve performance in such patients. Methods. We retrospectively enrolled 30 consecutive patients with symptomatic high-grade aortic stenosis scheduled for transfemoral TAVI between October 2019 and May 2020. Comprehensive computed tomography angiography for TAVI planning included standard measurements and quantification of calcification of the aortic valve. All patients had an aortic annular size in proximity to the valve size (maximum <15%) and received an S3U valve. Before discharge, paravalvular leakage was assessed via transthoracic echocardiography with an operator blinded to the TAVI results. In addition, 30-day outcome was assessed. Results. The S3U was implanted in all patients without any procedural complications. One patient received a 20 mm S3U valve, 18 received 23 mm S3U valves, and 11 received 26 mm S3U valves; the annular sizes were 19.7 mm, 22.9 ± 0.2 mm, and 25.8 ± 0.2 mm, respectively. Quantification of calcification of the aortic valve revealed significant calcifications with a median Agatston score of 2571 AU (interquartile range, 1685-3467 AU). Postprocedural transthoracic echocardiography showed an excellent result in all but 2 patients. In the latter, aortic insufficiency grade I was seen. Thirty-day survival was 96.7%. Conclusions. The new S3U valve shows excellent performance in patients with high-grade aortic stenosis and annular size in proximity to the valve size, even in presence of significant valvular calcification.
J INVASIVE CARDIOL 2021;33(5):E344-E348. Epub 2021 March 17. doi:10.25270/jic/20.00553
Key words: annular size, paravalvular leakage, Sapien 3 Ultra, transcatheter aortic valve implantation
Since the introduction of transcatheter aortic valve implantation (TAVI) therapy, there has been substantial improvement in the technique, including reduction in sheath size as well as valve modifications to prevent paravalvular leakage (PVL).1 Several large, randomized studies have shown efficacy of TAVI not only in elderly high-risk groups but also in younger medium-to-low risk cohorts.2 Despite these convincing data, treatment of TAVI patients remains challenging in certain subgroups.3 One of these subgroups comprises patients with an aortic annular size in proximity to the selected valve size. In such patients, the selected valve may be undersized with the potential for PVL and subsequently worse outcome. The new Sapien 3 Ultra (S3U) TAVI system (Edwards Lifesciences) offers the potential for more PVL prevention due to an improved valve design. The aim of this study was to assess the performance of the S3U valve in patients with high-grade aortic stenosis and an annular size in proximity to the size of the selected TAVI system.
Methods
We retrospectively enrolled 30 consecutive patients with symptomatic high-grade aortic stenosis scheduled for transfemoral TAVI between October 2019 and May 2020. In all patients, significant epicardial stenoses were ruled out by invasive angiography before TAVI. All patients received contrast-enhanced computed tomography angiography (Siemens Somatom Definition Flash or Canon Aquilion One Vision Edition) for TAVI planning. Comprehensive computerized analysis (using Syngovia software) included standard measurements as well as quantification of aortic valve and left ventricular outflow tract calcifications, as reported previously.4 All patients had an aortic annular size in proximity to the respective size of the new valve. This was defined as a maximum of 15% of the annulus diameter below the respective valve size. All patients were treated with an S3U TAVI system using a transfemoral approach in analgosedation. The decision to perform balloon valvuloplasty was left to the discretion of the operator. Moreover, the operator could decide whether or not the TAVI system was filled with more volume either at the initial implantation or at postdilation. The immediate postinterventional result was assessed by aortography using 30 mL of contrast with an injection speed of 15 mL/sec. Before discharge, the new aortic valve prosthesis was assessed via transthoracic echocardiography with an operator blinded to the TAVI data. In addition, 30-day survival was assessed via telephone calls or electronic patient records. The study complied with the Declaration of Helsinki and its later amendments and was in accordance with local ethics committee regulations. Data analysis was performed utilizing SPSS, version 22.0 (IBM). Baseline continuous variables are presented as mean ± standard deviation or median with interquartile range (IQR), depending on the distribution of the data. Categorical variables are presented as counts and percentages.
Results
The patient cohort was representative of a typical TAVI cohort, with a mean age of 82 ± 6 years and mean EuroSCORE II of 4.3% (Table 1). Quantification of aortic valve and left ventricular outflow tract calcification revealed a median Agatston score of 2571 AU (IQR, 1685-3467 AU) for the aortic valve and 112 AU (IQR, 11-293 AU) for the left ventricular outflow tract (Figure 1). The annular sizes were 19.7 mm, 22.9 ± 0.2 mm, and 25.8 ± 0.2 mm, respectively, for the 20 mm S3U device (n = 1 patient), 23 mm S3U (n = 18 patients), and 26 mm S3U device (n = 11 patients). In all 30 patients, the S3U was implanted without any procedural complications. Balloon valvuloplasty was performed before TAVI in 26 of the 30 patients with commercially available balloons. The decision for an increased balloon filling during TAVI was made in 23 of the 30 patients, as described previously for the Edwards Sapien 3 valve(Table 2).5 Postdilation was performed in 4 of the 30 patients (13%). Immediate postprocedural aortography revealed an excellent result in 25 patients (83%) and grade I aortic insufficiency in 5 patients (17%). Postprocedural transthoracic echocardiography (2-3 days after TAVI) showed an excellent result in all but 2 patients. Aortic insufficiency grade I was seen in the latter. Thirty-day outcome was successfully assessed in all 30 patients. Thirty-day survival rate was 96.7%; 1 patient passed away at day 8 post TAVI due to an unexpected arterial bleeding complication at the access site.
Discussion
This is the first study in the challenging group of TAVI patients with an annulus diameter in proximity to valve size, and shows that the S3U TAVI system provides excellent results. Increased balloon filling, as performed in 77% of all patients, was safe. To date, there have been no clear recommendations regarding the selection of the most appropriate TAVI system as well as the potential use of increased balloon filling above the nominal limit in these patients. The results of the present study support the use of the S3U in such patients, showing excellent performance and post-TAVI results. Moreover, the patients in our study had significant valvular calcifications, thus representing a real-world cohort of TAVI patients.
The problem in patients with an annulus size in proximity to the valve size is that the valve may be undersized, potentially leading to PVL and subsequently worse outcome. One possibility to overcome this challenge is to overextend the TAVI system, as previously described for the Sapien S3 valve.5 To the best of our knowledge, data regarding overextension of the S3U — as assessed in the present study — have not been reported to date. Another possibility is to select the next-higher valve size, with the potential hazard of severe oversizing and subsequent complications. In this context, the S3U offers the potential of more PVL prevention due to the improved valve design, including a ~40% increase in outer skirt height by maintaining the same inner skirt height compared with the S3 model. Moreover, the outer skirt consists of a new, textured, polyethylene terephthalate material, which is biocompatible (similar to the S3 model). Due to the fact that the S3U has only been commercially available since November 2018, there are limited clinical data. However, a recent international study on transfemoral TAVI using the S3U showed excellent in-hospital and 30-day outcomes.6 The results of this study are encouraging, and larger series confirming excellent safety and performance data in patients receiving an S3U are eagerly awaited.
Study limitations. The results of our study should be interpreted with caution, as we studied a small sample size at a single center. Moreover, TAVI patients with different clinical and anatomic/morphologic profiles may have different results when an S3U valve is chosen. Nevertheless, we believe that the results of our study are important, as the new valve design may contribute to a reduction in PVL, especially in the type of patients in the present study cohort.
Conclusion
The new S3U TAVI valve shows excellent performance in patients with high-grade aortic stenosis and annular size in proximity to the valve size even in presence of significant valvular calcifications.
From the Departments of 1Cardiology and Angiology, 2Cardiovascular Surgery, 3Radiology, and 4Anesthesia and Operative Intensive Care, Robert-Bosch-Krankenhaus, Stuttgart, Germany.
Funding: This study was done with support from the Berthold-Leibinger-Foundation.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
The authors report that patient consent was provided for publication of the images used herein.
Manuscript accepted September 24, 2020.
Address for correspondence: Peter Ong, MD, Robert-Bosch-Krankenhaus, Department of Cardiology, Auerbachstr. 110, 70376 Stuttgart, Germany. Email: Peter.Ong@rbk.de
- Hamm CW, Arsalan M, Mack MJ. The future of transcatheter aortic valve implantation. Eur Heart J. 2016;37:803-810.
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695-1705.
- Barbanti M, Webb JG, Dvir D, Prendergast BD. Residual challenges in TAVI: moving forward. EuroIntervention. 2019;15:857-866.
- Eberhard M, Mastalerz M, Frauenfelder T, et al. Quantification of aortic valve calcification on contrast-enhanced CT of patients prior to transcatheter aortic valve implantation. EuroIntervention. 2017;13:921-927.
- Shivaraju A, Kodali S, Thilo C, et al. Overexpansion of the Sapien 3 transcatheter heart valve: a feasibility study. JACC Cardiovasc Interv. 2015;8:2041-2043.
- Saia F, Gandolfo C, Palmerini T, et al. In-hospital and thirty-day outcomes of the Sapien 3 Ultra balloon-expandable transcatheter aortic valve: the S3U registry. EuroIntervention. 2020;15:1240-1247.