ADVERTISEMENT
Percutaneous Closure of the Patent Ductus Arteriosus Using the Nit-Occlud PDA-R (Reverse) Device: Initial Experience Reporting Immediate and Short-Term Results
Abstract: Purpose. To review the initial clinical outcomes of patent ductus arteriosus (PDA) closure using the new Nit-Occlud PDA-R device (NOPDA-R). Materials and methods. The NOPDA-R is a self-expandable, nitinol-made, premounted and cone-shaped device with two distinctive features: reverse reconfiguration of the distal disc and a peculiar “snare-like” release mechanism. From May to December 2010, 20 consecutive patients were included. Results. Median age was 4.7 years (range, 6 months to 21 years) and weight was 16.4 kg (range, 6-49 kg). Mean PDA diameter at its narrowest point, usually the pulmonary end, was 2.92 ± 0.61 mm (range, 2.1-4.5 mm), length was 7.05 ± 1.17 mm (range, 4.7-9.2 mm) and diameter of the aortic ampulla was 9.52 ± 1.62 mm (range, 6-13 mm). Pulmonary artery mean pressure was 20.6 ± 4.49 mmHg (range, 14-28 mmHg) and Qp/Qs ratio was 2.0 ± 0.29 (range, 1.6-2.5). Implantation success rate was 100%. The median cylinder diameter of the device was 6.53 ± 1.05 mm (range, 5.5-8.5 mm) leading to a final selected device 124% larger (cylinder diameter) than the narrowest PDA diameter. Assessed by transthoracic color-Doppler echocardiography at 24 hours, 1 month, and 3 months after implantation, complete closure was achieved in 60%, 90%, and 95% of patients, respectively. There were no complications and all patients were discharged home the next day. Conclusion. Percutaneous PDA closure using the new NOPDA-R device was feasible, safe, and effective. Longer follow-up time and a larger number of patients are required to assess long-term performance.
J INVASIVE CARDIOL 2011;23(12):513-516
Key words: congenital heart disease, patent ductus arteriosus, device closure, Nit-Occlud PDA-R
______________________________________
Percutaneous occlusion of the patent ductus arteriosus (PDA) has been performed for almost 45 years utilizing a variety of different devices.1 The Porstmann plug, the Rashkind umbrella device, the Buttoned device, the Gianturco coil (Cook, Inc.), the Flipper detachable coil (Cook, Inc.), the Amplatzer ductal occluder versions I & II (AGA Medical-St. Jude Medical), and the Nit-Occlud coil (pfm Medical) have been utilized over the years with a high degree of implantation success and low incidence of complications, including residual shunting, hemolysis, and/or malposition.2-7 Since the early 90s, based on the accumulated experience, percutaneous closure has become the procedure of choice for PDA occlusion outside of the neonatal period. Nevertheless, investigation and development of new devices suitable for premature infants and neonates are underway.8
Although most of the devices available for use are safe and effective, some drawbacks still persist, including the need for large delivery catheters/sheaths for implantation precluding their use in small infants, cumbersome and technically challenging delivery mechanisms, the inability to reposition or recapture the device once it has been deployed, and the persistence of residual shunting. To overcome such problems, in recent years, newer devices have been utilized. The Nit-Occlud® PDA-R (NOPDA-R; pfm Medical) was developed with distinctive features with the goal to offer the interventionalist another option for PDA closure. This paper reports the first clinical experience with this new device describing the immediate and short-term outcomes from a single center.
Materials and Methods
 PDA-R device. The NOPDA-R is a self-expandable, premounted, mushroom- or cone-shaped device knitted from a single nitinol wire without any soldering or protruding clamps or screws. It is intended for closure of PDAs measuring between 2 and 8 mm in diameter, offering the capability of recapturing and repositioning before release (Figure 1).
PDA-R device. The NOPDA-R is a self-expandable, premounted, mushroom- or cone-shaped device knitted from a single nitinol wire without any soldering or protruding clamps or screws. It is intended for closure of PDAs measuring between 2 and 8 mm in diameter, offering the capability of recapturing and repositioning before release (Figure 1).
Although the device characteristics, such as the nitinol frame work, loading maneuver, delivery technique, and deployment are similar to other self-expandable devices, the NOPDA-R has a distinct design based on two aspects: “reverse” reconfiguration of the distal retention disc and the “snare-like” release mechanism. The retention disc shows a unique “reverse” reconfiguration that fixes the implant to the aortic ampulla while the cylindrical structure expands against the wall of the vessel to stabilize the device in position. The retention disc is 4 to 7 mm larger in size than the diameter of the device. To improve its closure rate, a polyester membrane is sewn onto the center of the distal disc and into the cylinder itself to induce thrombosis. Two platinum markers are applied to the wire ends for radiological guidance during implantation.
The release mechanism includes a central “locking wire” that crosses the device entirely and a “pusher” with a distal wire noose (“eyelet”). The locking wire is attached to the implant by 4 accessory wires connected to the pusher. For release, a distal “security seal” is removed and the “locking wire” is retracted, disengaging the noose and freeing the implant.
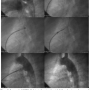 The device is available in sizes ranging from 4 to 13 mm in cylinder diameter, 6.5 to 13.5 mm in length, and 8 to 20 mm in final retention disc diameter. They require 5 to 9 Fr Mullins introducing sheaths (Cook, Inc.) for delivery. There are 7 commercially available device sizes numbered according to the minimal PDA diameter to be occluded. The selection of the device is dictated by measurements of the minimal PDA diameter (usually at the pulmonary artery end), the aortic ampulla diameter, and the ductal length (between the aortic and pulmonary end). The recommended diameter of the selected device must be at least 1.5 to 2 times greater than the minimum diameter of the defect. Figure 2 demonstrates the steps of the closure.
The device is available in sizes ranging from 4 to 13 mm in cylinder diameter, 6.5 to 13.5 mm in length, and 8 to 20 mm in final retention disc diameter. They require 5 to 9 Fr Mullins introducing sheaths (Cook, Inc.) for delivery. There are 7 commercially available device sizes numbered according to the minimal PDA diameter to be occluded. The selection of the device is dictated by measurements of the minimal PDA diameter (usually at the pulmonary artery end), the aortic ampulla diameter, and the ductal length (between the aortic and pulmonary end). The recommended diameter of the selected device must be at least 1.5 to 2 times greater than the minimum diameter of the defect. Figure 2 demonstrates the steps of the closure.
Patients. From May 2010 to December 2010, a total of 20 patients (15 female) were evaluated and fulfilled inclusion criteria to undergo an attempt of anterograde transcatheter closure of a PDA using the NOPDA-R as an alternative to standard use of other available coils/devices. The device was approved for clinical use by ANMAT (local medical devices approval authority). Informed consent was obtained from all patients or their guardians. All patients had clinical and echocardiographic findings of a hemodynamically significant PDA with increased left ventricular end diastolic dimensions. Exclusion criteria included a weight less than 6.0 kg, pulmonary vascular resistance (Rp) greater than 8 Woods units, Rp/systemic vascular resistance 0.4, history of serious infection 1 month prior to the procedure, or associated cardiac anomalies that would require cardiac surgery. None of the evaluated patients were excluded.
Closure and follow-up protocol. Under general endotracheal anesthesia, the femoral vein and femoral artery were cannulated and heparin sulphate given (100 IU/kg). After standard right and left catheterization, an angiogram was obtained in straight left lateral projection (90º) using a pigtail catheter located in the distal aortic arch. If the PDA was not precisely delineated, an additional angiogram was performed in right anterior oblique view (45º). After defining the PDA morphology and dimensions, the defect was crossed in an antegrade fashion and an 0.035˝ exchange wire was placed in the distal descending aorta followed by a long Mullins sheath. Subsequently, the selected device was advanced through the long sheath and the distal reverse disc was carefully opened in the aorta at the level of the ductal region. The whole system was pulled back as a unit so that the distal reverse disc was anchored to the aortic ampulla. While keeping some tension on the wire, the sheath was retracted in order to deploy the cylinder within the narrowest ductal diameter at the pulmonary side. While the device was still attached to the delivery system, an angiogram was obtained for confirmation of proper positioning. The device was released by pulling the thin inner-wire out (“snare mechanism”) of the device. Figure 2 shows an aortic angiogram in straight lateral projection before and after device PDA closure. All patients received 3 doses of cephalosporin (50 mg/kg; max, 1 gram). A chest radiograph in the posteroanterior view was obtained to assess device position and a complete 2-D and color-Doppler echocardiogram was performed the following day prior to discharge. Clinical visits were scheduled at 1, 3, and 6 months after the procedure during follow-up. Serial transthoracic echocardiograms were also performed at the visits with special attention given to possible residual ductal flow and increased flow velocity in the left pulmonary artery and descending aorta.
Collection of data and statistical analysis. Demographic and clinical data as well as the echocardiogram and catheterization reports on each patient were reviewed and prospectively collected from our database. Results are expressed as mean ± standard deviation or median and range. Statistical analyses were performed using the SPSS 12.0 (SPSS Institute, Inc.).
Results
 Table 1 shows demographic and clinical data on the patients enrolled. The median age was 4.7 years (range, 6 months to 21 years) and the median weight was 16.4 kg (range, 6-49 kg). The ductal types based on the angiographic Krichenko classification9 were as follows: Type A, 16 pts (80%); Type E, 2 pts (10%); and Type C and D, 1 pt each (5% each). The mean PDA diameter at its narrowest point, usually at the pulmonary end, was 2.92 ± 0.61 mm (range, 2.1-4.5 mm), the mean length was 7.05 ± 1.17 mm (range, 4.7-9.2 mm) and the mean diameter of the aortic ampulla was 9.52 ± 1.62 mm (range, 6-13 mm). The median pulmonary artery mean pressure was 20.6 ± 4.49 mmHg (range, 14-28 mmHg) and the mean Qp/Qs ratio was 2.0 ± 0.29 (range, 1.6-2.5). The implantation success rate was 100%. The median fluoroscopy time was 7.12 min (range, 5-13 min) and the median total procedure time was 42 min (range, 20-77 min). The median cylinder diameter of the device was 6.53 ± 1.05 mm (range, 5.5-8.5 mm) leading to a final selected device 124% larger (cylinder diameter) than the narrowest PDA diameter. It is worth mentioning that after an initial device-size was selected, none of the devices had to be changed.
Table 1 shows demographic and clinical data on the patients enrolled. The median age was 4.7 years (range, 6 months to 21 years) and the median weight was 16.4 kg (range, 6-49 kg). The ductal types based on the angiographic Krichenko classification9 were as follows: Type A, 16 pts (80%); Type E, 2 pts (10%); and Type C and D, 1 pt each (5% each). The mean PDA diameter at its narrowest point, usually at the pulmonary end, was 2.92 ± 0.61 mm (range, 2.1-4.5 mm), the mean length was 7.05 ± 1.17 mm (range, 4.7-9.2 mm) and the mean diameter of the aortic ampulla was 9.52 ± 1.62 mm (range, 6-13 mm). The median pulmonary artery mean pressure was 20.6 ± 4.49 mmHg (range, 14-28 mmHg) and the mean Qp/Qs ratio was 2.0 ± 0.29 (range, 1.6-2.5). The implantation success rate was 100%. The median fluoroscopy time was 7.12 min (range, 5-13 min) and the median total procedure time was 42 min (range, 20-77 min). The median cylinder diameter of the device was 6.53 ± 1.05 mm (range, 5.5-8.5 mm) leading to a final selected device 124% larger (cylinder diameter) than the narrowest PDA diameter. It is worth mentioning that after an initial device-size was selected, none of the devices had to be changed.
There were no complications and all patients were discharged home the following day. Complete closure was observed in 12 pts (60%) prior to discharge as assessed by transthoracic echocardiography with color Doppler. This rate increased to 90% (18 pts) and 95% (19 pts) at 1- and 3-month visit, respectively. The remaining patient showed a small leak (less than 1 mm) around the device (at the superior aspect) at the 3-month follow-up visit. No patient showed increased flow velocities in the left pulmonary artery and in the descending aorta.
Discussion
Over the past four decades, several different coils/devices and techniques have been described for percutaneous closure of PDA. Currently, there is no general consensus for selection of the best coil/device and technique in terms of safety, efficacy, and cost-effectiveness for percutaneous PDA occlusion. Part of the explanation lays on the great variability of ductal morphologies and sizes to preclude the use of a single coil/device for closure. Furthermore, there has been off-label use of devices that were not specifically designed for this lesion and are utilized either in very unusual ductal anatomies or in patients with high pulmonary artery pressures.10,11
The NOPDA-R is a premounted, user-friendly device, with a simple and efficient delivery system and release mechanism, allowing for recapturing and repositioning several times before release. It requires small introducer sheaths for the smaller-diameter devices and achieves a high closure rate. The sturdier distal retention disc, due to its peculiar reverse configuration, offers a secure anchoring mechanism to the aortic ampulla, minimizing the risks of “pulling-through” during implantation or inadvertent embolization after release. Careful reverse distal disc opening is advised in order to prevent any posterior aortic wall injury.
Interestingly in our experience, the selected final device cylinder diameter was slightly more than twice the narrowest PDA diameter. Due to its soft nitinol wire mesh, we think it is possible and perhaps necessary to oversize the device until a clear “bow-tie” appearance is visualized on the device’s body. The polyester fiber membranes have a physical barrier effect, leading to thrombus formation until full endothelialization.
We have occluded all angiographic types of PDAs except type B. In this preliminary experience, the device seemed to be versatile, efficacious, and adaptable to the most frequent PDA morphologies.
In this small series, the prevalence of residual shunting was very low. The only patient who showed a persistent superiorly located small leak around the device at 3-month follow-up was a 23-year-old female with a large, type-A PDA with a pulmonary artery end measuring 4.5 mm in whom an 11.5 mm cylinder diameter device was implanted. The indentation in the cylinder of the implanted device was mild, and in retrospect, we feel that taking into consideration that the patient presented also a large and well-developed aortic ampulla, we should have chosen a bigger device to better cover the defect.
Regarding clinical outcomes, all devices were implanted as planned and the procedures finalized without complications. Furthermore, all patients were discharged home the next day after being hospitalized for just 24 hours. The median fluoroscopy time of 7.12 minutes and the median procedural time of 42 minutes compare favorably with previous catheterization series for this type of intervention.12
Neither aortic arch obstruction nor left pulmonary artery stenosis were detected immediately or during follow-up. Nevertheless, during follow-up we routinely evaluated the patients using only color-Doppler echocardiography. This strategy has been recently questioned by Kharouf et al,13 and according to their experience, patients undergoing PDA device occlusion should be investigated using lung perfusion radionuclide scintigraphy to assess perfusion of the left lung. They reported a significant risk of decreased perfusion to the left lung after device occlusion of the PDA, although most of the lesions were graded as mild. No correlation was found comparing this finding with echocardiographic data or hemodynamic direct measurement. They believe that Doppler echocardiography may not be a sensitive tool for detecting left pulmonary artery obstruction caused by devices.
Finally, one of the strengths of this small report is the inclusion of 20 consecutive patients irrespective of ductal type morphologies referred to our service for percutaneous PDA closure. Only the defect size (between 2 to 8 mm minimal diameter) was taken into consideration for selection of the NOPDA-R as an alternative to the standard use of other available coils/devices. Neither randomization nor selection of patients according to ductal angiographic types was performed. Of course, further experience is required including larger number of patients, broader diameter ranges and ductal morphologies to draw stronger conclusions.
Conclusions
In this initial clinical experience, percutaneous closure of hemodynamically significant PDAs using the new NOPDA-R device was feasible, safe, and effective. Longer follow-up time and a larger number of patients are required to assess its ultimate clinical performance and efficacy profile. Clinical trials are underway.
Acknowledgments. The authors wish to thank Dr. Carlos A.C. Pedra for his critical review of the manuscript.
References
- Portsmann W, Wierny L, Warnke H. Closure of persistent ductus arteriosus without thoracotomy. Ger Med Mon. 1967;12(6):259-261.
- Rashkind WJ, Causo CC. Transcatheter closure of patent ductus arteriosus: successful use in a 3.5 kilogram infant. Pediatr Cardiol. 1979;1:3-7.
- Cambier PA, Kirby WC, Wortham DC, Moore JW. Percutaneous closure of the small (less than 2.5 mm) patent ductus arteriosus using coil embolization. Am J Cardiol. 1992;69(8):815-816.
- Moore JW, George I, Kirkpatrick SE, et al. Percutaneous closure of the small patent ductus arteriosus using occluding spring coils. J Am Coll Cardiol. 1994;23(3):759-765.
- Hijazi Z, Geggel R. Results of anterograde transcatheter closure of patent ductus arteriosus using single or multiple Gianturco coils. Am J Cardiol. 1994;74(9):925-929.
- Mazura J, Walsh KP, Thanopoulos B, et al. Catheter closure of moderate-to large-sized patent ductus arteriosus using the new Amplatzer Duct Occluder: immediate and short-term results. J Am Coll Cardiol. 1998;31(4):878-882.
- Rao P, Kim S, Rey C, Onorato E, Sideris EB. Results of transvenous buttoned device occlusion of patent ductus arteriosus in adults. Am J Cardiol. 1998;131(6):368-373.
- Roberts P, Adwani S, Archer N, Wilson N. Catheter closure of the arterial duct in preterm infants. Arch Dis Child Fetal Neonatal. 2007;92(4):F248-F250.
- Krichenko A, Benson LN, Burrows P, Moes CA, McLaughlin P, Freedom RM. Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol. 1989;90(12):2525-2529.
- Zabal C, García-Montes J, Buendía-Hernández A, et al. Percutaneous closure of hypertensive ductus arteriosus. Heart. 2010;96(8):625-629.
- Pedra CA, Sanches SA, Fontes VF. Percutaneous occlusion of the patent ductus arteriosus with the Amplatzer device for atrial septal defect. J Invasive Cardiol. 2003;15(7);413-417.
- Pass R, Hijazi Z, Hsu D, Lewis V, Hellenbrand WE. Multicenter USA Amplatzer Patent Ductus Arteriosus occlusion device trial. Initial one-year results. J Am Coll Cardiol. 2004;44(3):513-519.
- Kharouf R, Heitschmidt M, Hijazi Z. Pulmonary perfusion scans following transcatheter patent ductus arteriosus closure using the Amplatzer devices. Catheter Cardiovasc Interv. 2011;77(5):664-670.
______________________________________
From the Hospital Privado Centro Médico de Córdoba, Córdoba, Argentina.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted July 25, 2011, provisional acceptance given August 8, 2011, final version accepted August 29, 2011.
Address for correspondence: Dr. Alejandro R. Peirone, Head, Division of Cardiology, Hospital Privado de Córdoba, Naciones Unidas 346, Córdoba, Córdoba 5016, Argentina. Email: alepeirone@yahoo.com












