Pacemaker-Related Complications in Patients Undergoing Transcatheter Aortic Valve Implantation: A Single-Center Experience
Abstract: Background. The aim of this study was to describe the rate of complications secondary to permanent pacemaker (PPM) implantation after transcatheter aortic valve implantation (TAVI). Methods. Patients were identified by retrospective review of a dedicated TAVI database at a single center between 2008 and 2015. Clinical and procedural data were collected to determine the incidence and severity of the main adverse events following this procedure. The overall population included 736 patients and 123 (16.7%) underwent PPM implantation. Three patients were excluded from the final analysis due to PPM implantation occurring at other institutions post discharge. The final population consisted of 120 patients (16.3%). Results. Self- and mechanically expandable valves were associated with a higher risk of PPM after TAVI compared with balloon-expandable valves (23.9% vs 27.5% vs 7.6%, respectively; P<.001). Year of procedure and operator’s experience did not affect the PPM-related complication rate. A high rate of major bleeding complications (n = 29; 24.1%) was observed. Major bleeding occurred more frequently in patients who received PPM implantation within the first 24 hours post TAVI than in the group of patients who required a PPM >24 hours post TAVI (38.2% vs 12.3%, respectively; P<.01). Patients who experienced a major bleeding event following PPM insertion were more frequently on triple-antithrombotic therapy (51.7% vs 9.9%; P<.001). Conclusions. PPM-related complications are common in elderly patients after TAVI, and some of these adverse events can be life threatening. Particular attention is required in the periprocedural management of these patients.
Key words: atrioventricular block, bleeding, clinical research, conduction abnormalities, TAVI
A permanent pacemaker (PPM) is often required following transcatheter aortic valve implantation (TAVI), with an overall implantation rate of about 15% (~25% and 7% following TAVI with self-expanding and balloon-expandable valves, respectively).1 Approximately 75% of PPMs are implanted in the periprocedural period for new-onset high-degree or complete atrioventricular block.2 In the elective setting, PPM implantation is a routine procedure with a relatively low complication rate; different rates of early complications (ie, in hospital and at 30-day follow-up) are described in the literature3 and vary from 4.5%-12.6%.3,4 PPM implantation after TAVI could be more challenging and hazardous due to patient characteristics, such as older age, the high burden of comorbidities in the TAVI population, and the administration of periprocedural antithrombotic agents. Moreover, the risk of bleeding in this population in the perioperative period could be increased due to gastrointestinal angiodysplasia and change in factor VIII coagulant activity (Heyde’s syndrome).5
However, data about PPM-related complications in this population are lacking. The aim of this analysis was therefore to assess the rate of complications secondary to PPM implantation after TAVI.
Methods
Design and study population. A retrospective review of a single-center dedicated TAVI database was performed by 4 cardiologists in order to identify patients who underwent in-patient PPM implantation after TAVI. All consecutive TAVI patients between 2008 and 2015 were included and PPM-related major complications were analyzed. Indications for PPM implantation are shown in Table 1. All patients received intravenous antibiotic prophylaxis before the procedure according to the European Society of Cardiology guidelines.6 The management of the antiplatelet and anticoagulant therapy was left to the clinician’s discretion. All procedures were performed by expert cardiac electrophysiologists or by registrars (who had implanted <20 PPMs each) under consultant supervision7; postprocedural clinical examination, laboratory exams, and chest x-ray were routinely performed. After the procedure, patients were not mobilized and had a compression dressing over the PPM pocket site to reduce the rate of bleeding and lead detachment. All patients underwent a chest x-ray to check the correct position of PPM leads and to rule out pneumothorax. In the presence of a pocket hematoma, patients underwent cold topic application and the anticoagulant/antiplatelet therapy was discontinued when possible.
Antiplatelet/anticoagulant regimens. We reviewed all antiplatelet/anticoagulant medication during a time frame of ± 48 hours from the procedure. We reviewed all medical reports to identify the dosage and use of heparin and/or protamine during and after the TAVI procedure.8
Patients were divided into 6 groups: (1) patients who were not on antiplatelet or anticoagulant therapy; (2) patients on single-antiplatelet therapy (SAPT); (3) patients on dual-antiplatelet therapy (DAPT); (4) patients on dual therapy (ie, SAPT + anticoagulant); (5) patients on triple therapy (ie, DAPT + anticoagulant); and (6) patients on anticoagulant therapy. Clinical follow-up was regularly performed with telephone interviews or outpatient reviews.
Definitions and clinical endpoints. PPM-related complications were divided into the following categories:9 (1) Access-related complications. Access complications were defined as any complication that was vessel related or that occurred while gaining access to the vasculature or the device pocket (pneumothorax, hemothorax, hematoma — including need for transfusion — and thrombosis of venous or arterial vessels). Pocket hematoma was defined as the presence of any palpable mass or ecchymosis exceeding the size of the generator in the pocket area.10 (2) Device-related complications. Lead-related complications were defined as any complication caused by placement of the lead or subsequently related directly to the lead; for example, dissection or perforation (to include tamponade), displacement, fracture, undersensing or oversensing, insulation defects, and the need for any lead revision regardless of cause. Generator-related complications were defined as generator failure and any need for generator revision (for example, migration, or erosion) but excluding infection (classified separately). (3) Infection complications were defined as infections requiring intervention, such as antibiotic therapy or device extraction.
Our analysis sought to describe the rate of PPM-related complications, focusing on the rate of major bleeding, according to the Valve Academic Research Consortium-2 criteria.11
Valve types. Aortic valves used during the procedures were divided into 3 main groups: (1) balloon-expandable valves, including Edwards Sapien, Sapien XT, and Sapien 3 (Edwards Lifesciences); (2) self-expanding valves, including CoreValve, CoreValve Evolut R, and Engager (Medtronic), Acurate neo (Boston Scientific), Direct Flow (Direct Flow Medical), Portico (St Jude Medical), and JenaValve (JenaValve Technology); and (3) mechanically expandable valves, including the Lotus valve (Boston Scientific)
Statistical analysis. Continuous variables are presented as mean ± standard deviation and differences and were compared with the unpaired Student’s t-test or Mann-Whitney U-test. Categorical variables are presented as numbers and percentages and were analyzed using the Chi-squared or Fisher’s exact tests. All reported P-values were 2-sided and a P<.05 was considered statistically significant. Multivariable logistic regression analysis was performed to identify independent predictors of major bleeding events. Results of the logistic regression analysis are presented as odds ratio (OR) with 95% confidence interval (CI). The statistical analyses were performed using SPSS, version 16.0.2 (SPSS).
Results
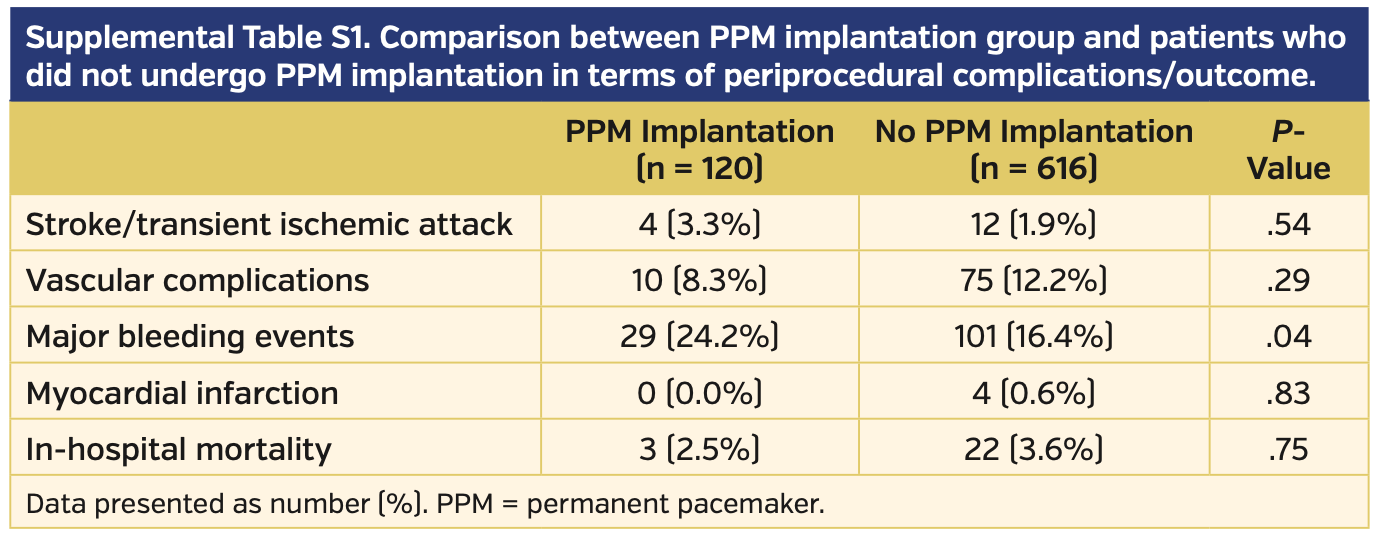
During the study period, a total of 736 consecutive patients underwent TAVI, with 3 patients (0.4%) excluded due to PPM implantation occurring at other institutions after discharge. No differences were found in terms of periprocedural complication rates and in-hospital mortality rates when comparing patients who underwent PPM implantation after TAVI with those who did not require a PPM, except for major bleeding events (Supplemental Table S1).
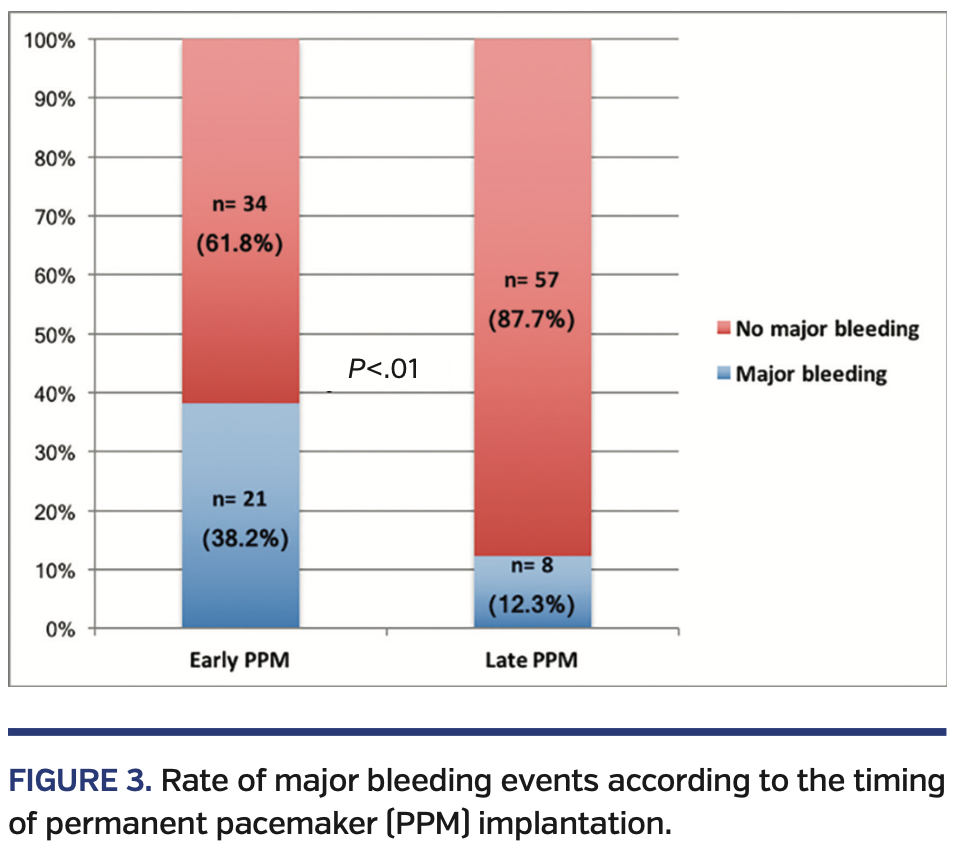
The study group included 120 patients (16.3%) undergoing PPM implantation following TAVI at our center. From these, 55 patients (45.8%) underwent PPM implantation during the first 24 hours and 65 patients (53.2%) had late PPM implantation (defined as >24 hours post TAVI). PPM-related complications were observed in 34 patients (28.3%) and were more frequent in those patients who had PPM implantation within the first 24 hours after TAVI than in those with late PPM implantation (23 patients [67.6%] vs 11 patients [32.3%], respectively; P<.01).
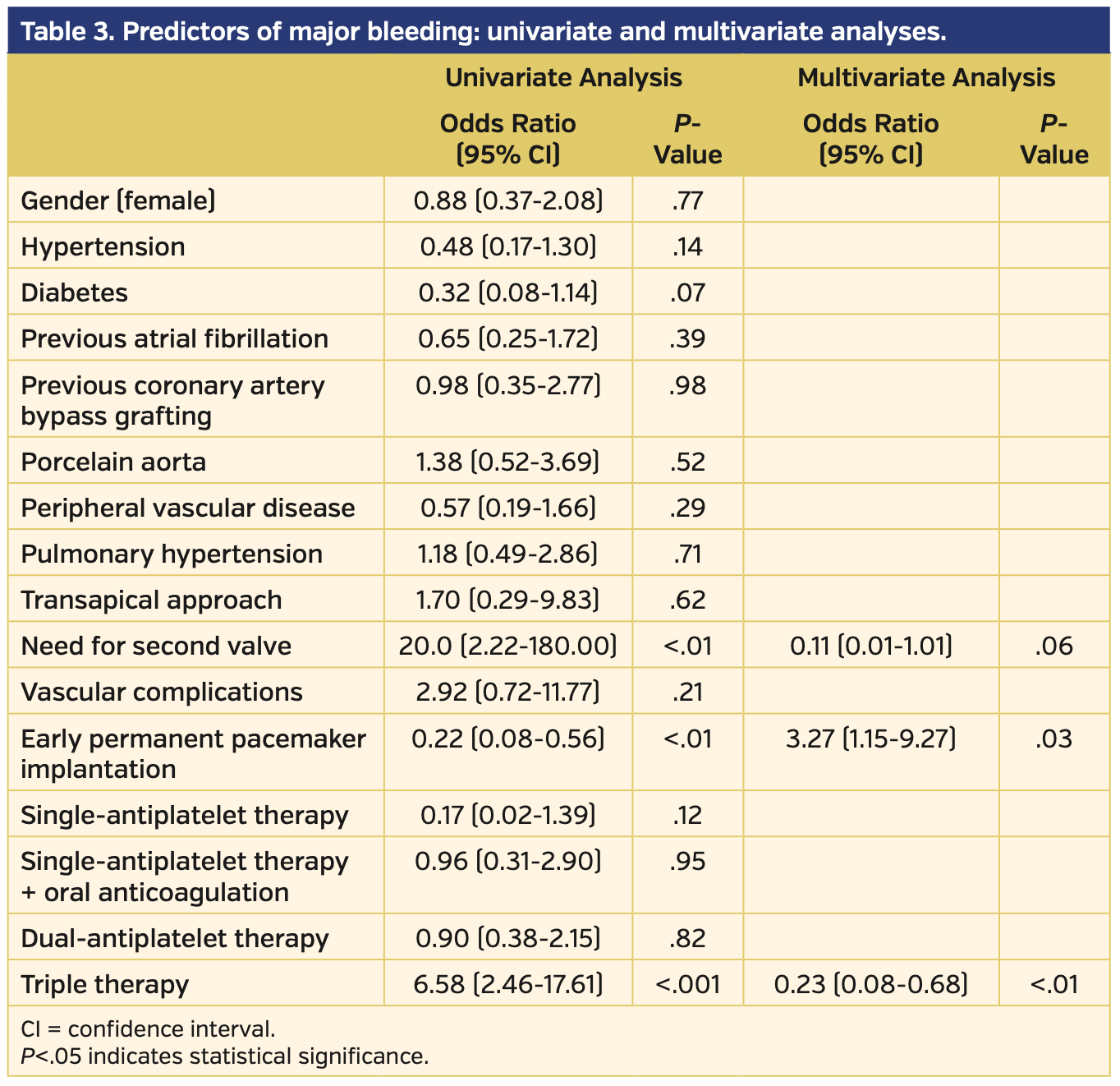
Baseline characteristics of the study population are shown in Table 2. No significant differences were found between patients with PPM-related complications vs those who did not present complications. As previously reported in the literature,12,13 self-expanding and mechanically expandable valves were associated with a higher risk of PPM after TAVI compared with balloon-expandable valves (23.9% vs 27.5% vs 7.6%, respectively; P<.001) (Figure 1).
PPM-related complications. Most PPM-related complications were access-type complications, which were reported in 29 patients (85.3%); all of these events turned out to be major bleeding events. Device-related complications were observed in 3 patients (8.8%), while an infective complication occurred in 2 patients (5.9%) (Figure 2).
In addition, 8 patients (6.7%) had fever after the procedure, but there was a direct correlation with an infection of the PPM pocket in only 2 of these patients (1.7%); in 1 of these patients (0.8%), a pocket hematoma was observed as well. At follow-up, 2 patients (1.7%) died as a consequence of lead endocarditis.
Three patients (2.5%) had lead malfunction (2 patients required lead replacement and the other patient was managed successfully by reprogramming the PPM). Three PPM implantations (2.5%) were complicated by cardiac tamponade; 2 patients (1.7%) required percutaneous drainage, while 1 patient (0.8%) required a surgical drainage, with complete recovery. One patient (0.8%) had a pneumothorax 48 hours after PPM implantation, which was successfully treated with chest drain insertion. One patient (0.8%) had a thrombosis of the left brachiocephalic vein that required anticoagulation therapy. One patient (0.8%) had a major vascular event — dissection of the left internal mammary artery — and consequent hemothorax, which led to patient death.
In patients who underwent late PPM implantation following TAVI, those who experienced a PPM-related complication had prolonged hospitalization vs patients who underwent a late PPM implantation without complications (19.3 ± 11.9 days vs 13.9 ± 6.2 days, respectively; P=.03).
Hospitalization was longer (but not statistically significant) comparing patients who had a PPM-related complication vs those who did not in the group that underwent early implantation (12 ± 5.2 days vs 9.8 ± 3.5 days, respectively; P=.07) and in the overall population — ie, early and late PPM implantation (14.3 ± 8.5 days vs 12.4 ± 5.7 days, respectively; P=.15).
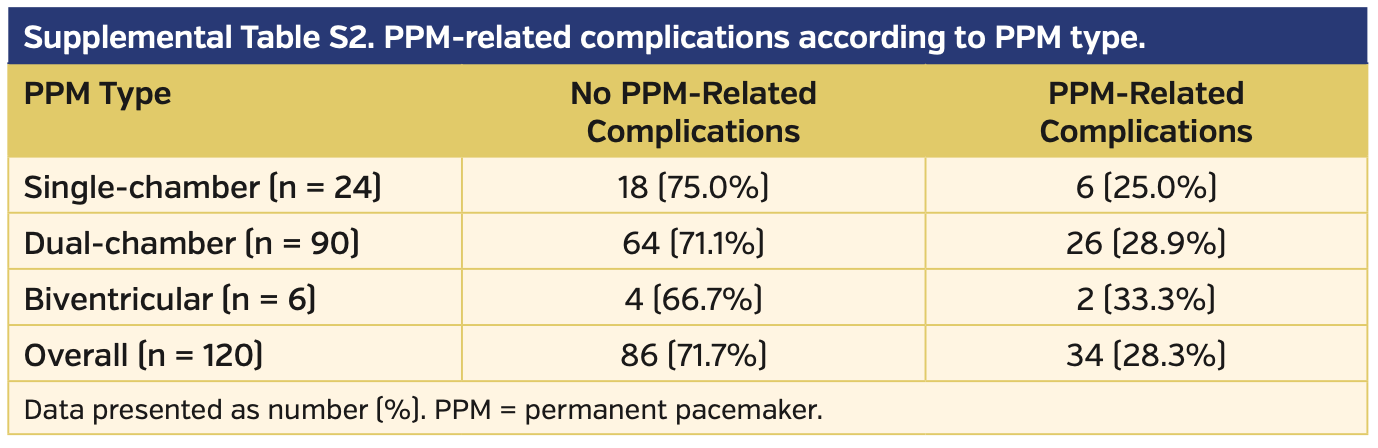
Comparing PPM types, no differences were observed in terms of complication rates between single-chamber, dual-chamber, and biventricular PPMs (P=.90) (Supplemental Table S2).
No statistically significant differences were observed in terms of complications based on year of the procedure (P=.26).
Bleeding events. A high rate of major bleeding events was observed in the population that underwent PPM implantation (29 patients; 24.2%). In 14 patients (11.7%), a pocket hematoma was observed. In patients who did not require PPM implantation, the rate of major bleeding events was significantly lower (101 patients; 16.4%; P=.04).
Major bleeding occurred more frequently in those patients who received PPM implantation within the first 24 hours after TAVI than in the group of patients who required late PPM implantation after TAVI (21 patients [38.2%] vs 8 patients [12.3%]; P<.01) (Figure 3).
As predicted, patients who underwent late PPM implantation were more often (26 patients; 40%) not completely dependent on a temporary pacing wire and there was a statistically significant difference between the 2 groups (5 patients [9.1%] vs 26 patients [40%]; P<.001).
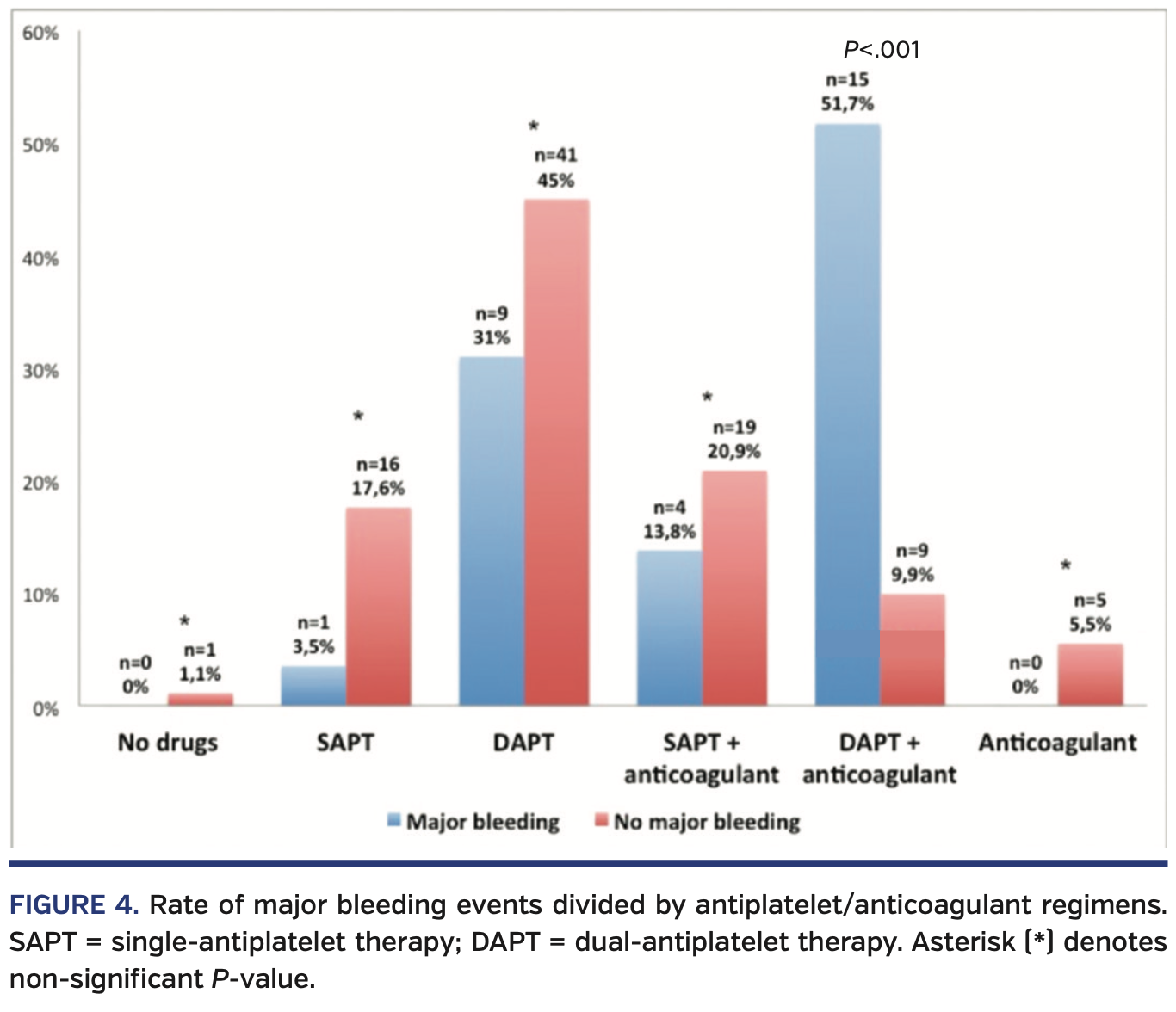
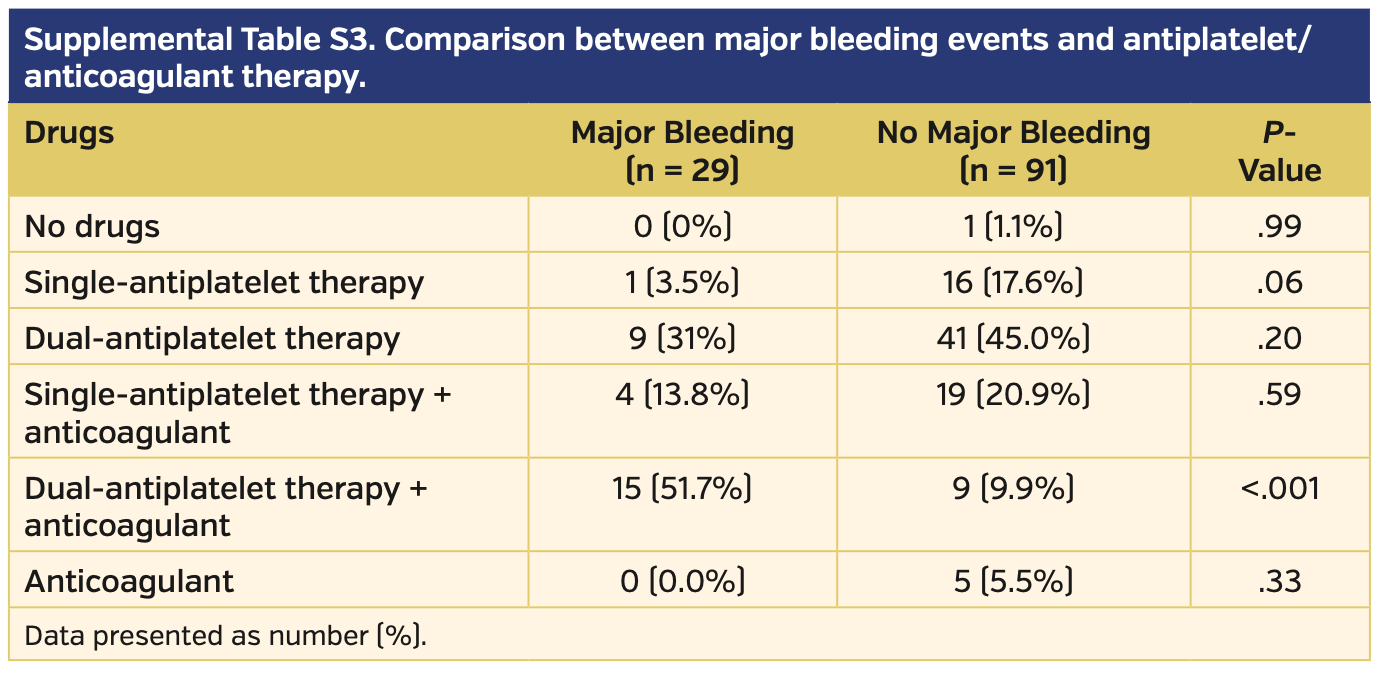
Patients who experienced a major bleeding event following the PPM insertion were more frequently on triple therapy vs those who did not experience bleeding complications (15 patients [51.7%] vs 9 patients [9.9%]; P<.001). These data were also confirmed with a comparison of anticoagulant/antiplatelet regimens between early vs late PPM implantation groups (patients who had early implantation were more frequently on triple therapy; 17 patients [30.9%] vs 7 patients [10.8%], respectively; P<.01).
Patients who underwent PPM implantation on SAPT showed a trend toward a reduction of major bleeding (1 patient [3.5%] vs 16 patients [17.6%], respectively; P=.06) (Supplemental Table S3). The rate of major bleeding was comparable in patients on other antiplatelet/anticoagulant regimens (Figure 4).
Discussion
As TAVI evolves toward treating patients with lower surgical risk and greater life expectancy, it is important to characterize common complications following this procedure. Despite technical improvements, the rate of PPM implantation remains high, with an overall incidence that ranges from 2%-51%.14-17
PPM has been demonstrated to have a possible detrimental effect on patient outcomes, increasing the rate of hospitalization and reducing the recovery of left ventricular ejection fraction.18 Moreover, PPM implantation is characterized by a non-negligible rate of complications, ranging from 7.4%-9.9%.10 The rate of PPM-related complications seems to be higher among patients >75 years old.19,20 This could be due both to the patients’ underlying comorbidities and to their antiplatelet/anticoagulant regimens,21 considering that in our population, among patients who received a PPM, only 17 patients (14.2%) were on SAPT and only 1 patient (0.8%) did not assume any antiplatelet/anticoagulant therapy (Supplemental Table S3).
To the best of our knowledge, this is the first report that describes the rate of PPM-related complications among the TAVI population. The main findings of this study are: (1) a high rate of PPM-related complications was observed in patients who underwent PPM implantation after TAVI, especially within the first 24 hours after TAVI; (2) patients on triple therapy were at higher risk of major bleeding events; and (3) patients who experienced a PPM-related complication had a longer hospitalization period, especially in the late PPM implantation group (defined as PPM >24 hours after TAVI) (Figure 2). Multivariable analysis confirmed that early PPM implantation and triple therapy were independent predictors of major bleeding events (Table 3).
We found that a PPM after TAVI was implanted in 16.3% of our population, and self-expanding and mechanically expandable valves were associated with a higher rate of implantation compared with balloon-expandable valves. The overall rate of PPM-related complications was 28.3% and mostly driven by major bleeding events (24.2%). Pacemaker infections and device-related complications were described in 5.9% and 8.8% of the population, respectively. In this scenario, leadless pacemakers may offer a valid alternative in the future, to eliminate lead- and pocket-associated complications.22
No differences were observed in PPM-related complication rates when comparing procedures performed by consultants with those performed by registrars, nor were differences observed when comparing PPM type. Despite the improvements in periprocedural management of TAVI procedures from 2008 to 2015, we did not find any significant temporal trend in PPM-related complications.
We observed a high rate of major bleeding, especially in those who had PPM implantation within the first 24 hours after TAVI. This finding is in line with the report by Bernelli et al,8 which showed that the final activated clotting time after TAVI correlates with the risk of bleeding events; the effect of heparin administered during TAVI can exacerbate bleeding, particularly in patients taking DAPT.
Data about major bleeding events observed in our population may have several clinical implications:
- Reversing anticoagulation by the administration of protamine at the end of the procedure could represent a safer strategy in order to minimize the risk of bleeding.
- A tailored approach should be recommended in order to prevent major adverse events in a population in which age and, more broadly, frailty play an important role in the choice of the most appropriate antiplatelet strategy. In particular, triple therapy should be avoided in patients requiring a PPM. Moreover, the use of SAPT should be encouraged considering that the use of DAPT has not shown benefits in terms of hard clinical events.23,24
- PPM implantation should be delayed at least 24 hours after the procedure to reduce the risk of bleeding events; in this scenario, attention should be paid in order to identify patients who may recuperate atrioventricular conduction.25 Out of 55 early implantations, 5 patients (9.1%) could have had delayed PPM implantation. This finding highlights the importance of carefully evaluating PPM indication and its timing in order to reduce major bleeding risk.
Early discharge after TAVI could reduce medical costs and facilitate early recovery, especially when performed at high-volume centers.26-28 Our analysis showed that this strategy could be hindered by PPM implantation after TAVI, especially when a complication arises after the procedure. Patients who experienced a PPM-related complication had a longer hospitalization period than those who underwent a complication-free procedure; this difference was particularly remarkable in the group that underwent a late PPM implantation >24 hours after TAVI. Therefore, attention should be paid to the prevention and correct management of PPM-related complications in order to allow an earlier discharge after TAVI.
Study limitations. This study was retrospective and non-randomized in design, making the results mostly hypothesis generating. In addition, although populations were similar in multiple risk factors, we cannot deny the possibility of additional confounding factors that may have caused an imbalance between groups and influenced outcomes.
Conclusion
PPM-related complications are not uncommon after TAVI, especially in terms of major bleeding events. These complications have a negative impact in terms of morbidity, rehabilitation, and prolonged length of stay. Particular attention is required in the periprocedural management of these patients, and a tailored antithrombotic therapy is recommended to minimize the risk of bleeding events. Further studies are needed to confirm our findings.
*Joint first authors.
From the 1Division of Cardiology, IRCCS Policlinico San Matteo, Pavia, Italy; 2Interventional Cardiology Unit, GVM Care & Research Maria Cecilia Hospital, Cotignola, Ravenna, Italy; 3Interventional Cardiology Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy; 4Department of Cardiology, Hammersmith Hospital, Imperial College Healthcare NHS Trust, London, United Kingdom; 5Charité University Medical Care, Berlin, Germany; 6Electrophysiology Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy; and 7Department of Cardiology, Montefiore Medical Center, New York.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Latib reports consultant income from Medtronic. The remaining authors report no conflicts of interest regarding the content herein.
Final version accepted May 12, 2020.
Address for correspondence: Azeem Latib, MD, Department of Cardiology, Montefiore Medical Center, 111 East 210th Street, Bronx, NY 10467-2401. Email: alatib@gmail.com
- Rodés-Cabau J. Transcatheter aortic valve implantation: current and future approaches. Nat Rev Cardiol. 2011;9:15-29.
- Urena M, Webb JG, Tamburino C, Muñoz-García AJ, et al. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation. 2014;129:1233-1243.
- Kotsakou M, Kioumis I, Lazaridis G, et al. Pacemaker insertion. Ann Transl Med. 2015;3:42.
- Haug B, Kjelsberg K, Lappegård KT. Pacemaker implantation in small hospitals: complication rates comparable to larger centres. Europace. 2011;13:1580-1586.
- Vincentelli A, Susen S, Le Tourneau T, et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349:343-349.
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075-3128.
- Leong KM, Pollard C, Cooke CJ. Cardiology registrars and permanent pacemaker complication rates in a district general hospital—safety and service implications. Clin Med (Lond). 2014;14:34-37.
- Bernelli C, Chieffo A, Montorfano M, et al. Usefulness of baseline activated clotting time-guided heparin administration in reducing bleeding events during transfemoral transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2014;7:140-151.
- Ezzat VA, Lee V, Ahsan S, et al. A systematic review of ICD complications in randomised controlled trials versus registries: is our ‘real-world’ data an underestimation? Open Heart. 2015;2:e000198.
- Wiegand UK, LeJeune D, Boguschewski F, et al. Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy. Chest. 2004;126:1177-1186.
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33:2403-2418.
- Sathananthan J, Ding L, Yu M, et al. Implications of transcatheter heart valve selection on early and late pacemaker rate and on length of stay. Can J Cardiol. 2018;34:1165-1173.
- Mangieri A, Lanzillo G, Bertoldi L, et al. Predictors of advanced conduction disturbances requiring a late (≥48 H) permanent pacemaker following transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2018;11:1519-1526.
- Siontis GC, Jüni P, Pilgrim T, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J Am Coll Cardiol. 2014;64:129-140.
- Khatri PJ, Webb JG, Rodés-Cabau J, et al. Adverse effects associated with transcatheter aortic valve implantation: a meta-analysis of contemporary studies. Ann Intern Med. 2013;158:35-46.
- Urena M, Webb JG, Tamburino C, et al. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation. 2014;129:1233-1243.
- Maeno Y, Abramowitz Y, Israr S, et al. Prognostic impact of permanent pacemaker implantation in patients with low left ventricular ejection fraction following transcatheter aortic valve replacement. J Invasive Cardiol. 2019;31:E15-E22.
- Nazif TM, Williams MR, Hahn RT, et al. Clinical implications of new-onset left bundle branch block after transcatheter aortic valve replacement: analysis of the PARTNER experience. Eur Heart J. 2014;35:1599-1607.
- Armaganijan LV, Toff WD, Nielsen JC, et al. Are elderly patients at increased risk of complications following pacemaker implantation? A meta-analysis of randomized trials. Pacing Clin Electrophysiol. 2012;35:131-134.
- Mahapatra S, Bybee KA, Bunch TJ, et al. Incidence and predictors of cardiac perforation after permanent pacemaker placement. Heart Rhythm. 2005;2:907-911.
- Nichols CI, Vose JG. Incidence of bleeding-related complications during primary implantation and replacement of cardiac implantable electronic devices. J Am Heart Assoc. 2017;6:e004263.
- Kancharla K, Deshmukh AJ, Friedman PA. Leadless pacemakers—implant, explant and long-term safety and efficacy data. J Atr Fibrillation. 2017;10:1581.
- Rodés-Cabau J, Masson JB, Welsh RC, et al. Aspirin versus aspirin plus clopidogrel as antithrombotic treatment following transcatheter aortic valve replacement with a balloon-expandable valve: the ARTE (aspirin versus aspirin + clopidogrel following transcatheter aortic valve implantation) randomized clinical trial. JACC Cardiovasc Interv. 2017;10:1357-1365.
- Mangieri A, Jabbour RJ, Montalto C, et al. Single-antiplatelet therapy in patients with contraindication to dual-antiplatelet therapy after transcatheter aortic valve implantation. Am J Cardiol. 2017;119:1088-1093.
- Gaede L, Kim WK, Liebetrau C, et al. Pacemaker implantation after TAVI: predictors of AV block persistence. Clin Res Cardiol. 2018;107:60-69.
- Barbanti M, Baan J, Spence MS, et al. Feasibility and safety of early discharge after transfemoral transcatheter aortic valve implantation — rationale and design of the FAST-TAVI registry. BMC Cardiovasc Disord. 2017;17:259.
- Alkhalil A, Lamba H, Deo S, et al. Safety of shorter length of hospital stay for patients undergoing minimalist transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2018;91:345-353.
- Tobin K, Stewart J, Westveer D, et al. Acute complications of permanent pacemaker implantation: their financial implication and relation to volume and operator experience. Am J Cardiol. 2000;85:774-776, A9.