Optical Coherence Tomography Analysis of the Stenting of Saphenous Vein Graft (SOS) Xience V Study: Use of the Everolimus-Eluting Stent in Saphenous Vein Graft Lesions
Abstract: Objectives. The Stenting of Saphenous Grafts-Xience V (SOS-Xience V) trial prospectively examined the frequency of angiographic in-stent restenosis in saphenous vein graft (SVG) lesions 12 months after implantation of a Xience V everolimus-eluting stent (EES; Abbott Vascular). Optical coherence tomography (OCT) during follow-up angiography was added to the protocol after OCT was approved for clinical use in the United States. Methods. Forty patients with 40 SVG lesions were enrolled in the study, of whom 27 underwent 12-month coronary angiography and 12 (only 1 of whom had in-stent restenosis) also had follow-up OCT evaluation. OCT strut-level analysis was performed to determine the percentage of strut coverage, malapposition, strut protrusion, neointimal thickness, and the existence of thrombus. Results. Mean patient age was 67 ± 7 years, and 95% were men. A total of 2584 struts were evaluated by OCT. The percentages for uncovered, malapposed, and protruding struts were 4%, 9%, and 15%, respectively. The mean strut neointimal thickness was 0.094 ± 0.094 mm. Of the 12 stents analyzed, 4 (33%) showed full neointimal coverage, 2 (17%) had all the struts embedded, 7 (58%) had at least 1 malapposed strut, and 10 (83%) had at least 1 protruding strut. The mean difference between the stent area and the lumen area was 0.36 ± 1.6 mm². No thrombus was detected in the stented areas. Conclusions. Use of EES in SVGs is associated with high rates of stent strut coverage and high malapposition rates at 12 months post implantation.
J INVASIVE CARDIOL 2012;24(8):390-394
Key words: saphenous vein grafts, drug-eluting stents, percutaneous coronary intervention, coronary artery bypass graft surgery, optical coherence tomography
_______________________________________________
Although two1-3 of the three published randomized-controlled trials of drug-eluting stents (DES) in saphenous vein grafts (SVGs) showed improved outcomes with DES implantation, concerns remain about the safety of DES in this setting, given the higher all-cause mortality observed with DES in the Reduction of Restenosis In Saphenous vein grafts with Cypher sirolimus-eluting stent (RRISC) trial.4 With a resolution of 15-20 µm, optical coherence tomography (OCT) may allow assessment of the risk of stent thrombosis by accurate evaluation of stent strut coverage and malapposition.5
The Stenting Of Saphenous Vein Grafts Xience V (SOS-Xience V) study (NCT00911976) was the first prospective study of a second-generation DES in de novo SVG lesions and showed 22% 12-month binary angiographic restenosis in patients receiving the Xience V everolimus-eluting stent (EES; Abbott Vascular) in SVG lesions.6 After approval of OCT imaging in the US, the SOS Xience V protocol was modified to incorporate OCT follow-up evaluation to determine the frequency of stent strut coverage and malapposition and evaluate neointimal thickness.
Methods
Study design and patients. The SOS-Xience V trial was a single-arm, non-randomized, open-label, prospective study that examined the 12-month angiographic and clinical outcomes of patients undergoing stenting of de novo SVG lesions using an EES. The primary study endpoint was binary angiographic in-stent restenosis, defined as stenosis of >50% of the minimum lumen diameter of the target SVG segment. The study enrolled 40 consecutive patients, 27 of whom underwent angiographic follow-up at 12 months. After OCT was approved for clinical use in the US, OCT evaluation at the time of follow-up angiography was added to the study protocol. The study was approved by the institutional review board of our institution.
Optical coherence tomography. OCT images were obtained by C7XR FD-OCT Imaging System (St Jude Medical) with a 2.7 Fr, 135-cm long coronary catheter introduced into the target SVG over a 0.014˝ guidewire. Automated mechanical pullback using a pullback and rotation device was performed at a speed of 20 mm/s during contrast injection, until the OCT catheter was withdrawn into the guiding catheter. The data were recorded on CDs and transferred to the LightLab Imaging Offline Review Workstation for strut-level analysis.
Measurements were made every third frame (0.6 mm intervals) along the entire stented area. A blooming was counted when it was accompanied by a shadow. Strut intimal thickness was measured based on automatic measurements from the center of the blooming to the lumen contour.7
Endpoints. The primary endpoints of the OCT substudy were: (a) stent strut coverage; (b) stent strut malapposition; and (c) neointimal thickness.
A strut was considered suitable for analysis only if it had both a well-defined bright “blooming” appearance and characteristic shadow perpendicular to the light source. The center of the luminal surface of the strut blooming was determined for each strut, and its distance to the lumen contour was calculated automatically to determine strut-level intimal thickness (SIT). Struts covered by tissue had positive SIT values, whereas uncovered or malapposed struts had negative SITs. The number of struts without coverage was counted for each frame analyzed, and the total number of frames with uncovered struts was recorded. Struts were classified as malapposed when the negative value of SIT was higher than 108 µm (81 µm strut thickness + 7.8 µm coating thickness + 20 µm resolution threshold for OCT).8,9
 Struts were classified in 6 categories: those not interrupting the smooth lumen contour that were covered by tissue were defined as embedded covered struts (Figure 1A); those not interrupting the smooth lumen contour, but not covered by tissue were defined as apposed uncovered struts (Figure 1B); those interrupting the smooth lumen contour (but were not malapposed) and were covered by tissue were defined as protruding covered struts (Figure 1C); those extending into the lumen (but were not malapposed) without tissue coverage
Struts were classified in 6 categories: those not interrupting the smooth lumen contour that were covered by tissue were defined as embedded covered struts (Figure 1A); those not interrupting the smooth lumen contour, but not covered by tissue were defined as apposed uncovered struts (Figure 1B); those interrupting the smooth lumen contour (but were not malapposed) and were covered by tissue were defined as protruding covered struts (Figure 1C); those extending into the lumen (but were not malapposed) without tissue coverage  were classified as protruding uncovered struts (Figure 1D); malapposed struts were classified as uncovered (Figure 1E) and covered (Figures 1F and 1G).
were classified as protruding uncovered struts (Figure 1D); malapposed struts were classified as uncovered (Figure 1E) and covered (Figures 1F and 1G).
Statistical analysis. Continuous parameters were presented as mean ± standard deviation, and nominal parameters were presented as percentages. All analyses were performed using JMP 9.0 software (SAS Institute).
Results
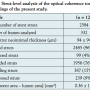
 Patients. The baseline characteristics of the study patients are shown in Table 1. Thirteen of the 40 study patients did not undergo follow-up angiography because of death (n = 6), moving to another state (n = 1), or because the patient declined (n = 6). After OCT system approval, follow-up OCT evaluation was performed in 12 patients, only 1 of whom had developed angiographic restenosis (Figure 2). Patients undergoing OCT evaluation had similar characteristics to those who did not (Table 1).
Patients. The baseline characteristics of the study patients are shown in Table 1. Thirteen of the 40 study patients did not undergo follow-up angiography because of death (n = 6), moving to another state (n = 1), or because the patient declined (n = 6). After OCT system approval, follow-up OCT evaluation was performed in 12 patients, only 1 of whom had developed angiographic restenosis (Figure 2). Patients undergoing OCT evaluation had similar characteristics to those who did not (Table 1).
 Angiographic and optical coherence tomography. At 12 months, binary angiographic restenosis occurred in 6 of 27 patients (22%) (95% confidence interval, 9%-42%) of EES-treated lesions. The pattern of restenosis was focal in 3 lesions (Figure 1H), diffuse intra-stent in 1 lesion, and occlusive in 2 lesions. The results of the OCT analysis are presented in Table 2 and Table 3. High rates of stent strut coverage were observed, in spite of high rates of stent strut malapposition (Figure 3). Four stents (33%) showed full neointimal coverage, 2
Angiographic and optical coherence tomography. At 12 months, binary angiographic restenosis occurred in 6 of 27 patients (22%) (95% confidence interval, 9%-42%) of EES-treated lesions. The pattern of restenosis was focal in 3 lesions (Figure 1H), diffuse intra-stent in 1 lesion, and occlusive in 2 lesions. The results of the OCT analysis are presented in Table 2 and Table 3. High rates of stent strut coverage were observed, in spite of high rates of stent strut malapposition (Figure 3). Four stents (33%) showed full neointimal coverage, 2  (17%) had all the struts embedded, 7 (58%) had at least 1 malapposed strut, and 10 (83%) had at least 1 protruding strut. The mean strut neointimal thickness in all patients was 0.094 ± 0.094 mm (interquartile range, 0.030-0.130 mm). The mean neointimal area was 0.36 ± 1.6 mm2. No thrombus was detected in the stented areas. The 1 in-stent restenotic lesion included in this OCT substudy demonstrated concentric neointimal hyperplasia with mean neointimal thickness of 0.2 ± 0.21 mm, mean lumen diameter of 2.7 ± 0.7 mm, and mean stent diameter of 3.01 ± 0.2 mm (Figure 1H).
(17%) had all the struts embedded, 7 (58%) had at least 1 malapposed strut, and 10 (83%) had at least 1 protruding strut. The mean strut neointimal thickness in all patients was 0.094 ± 0.094 mm (interquartile range, 0.030-0.130 mm). The mean neointimal area was 0.36 ± 1.6 mm2. No thrombus was detected in the stented areas. The 1 in-stent restenotic lesion included in this OCT substudy demonstrated concentric neointimal hyperplasia with mean neointimal thickness of 0.2 ± 0.21 mm, mean lumen diameter of 2.7 ± 0.7 mm, and mean stent diameter of 3.01 ± 0.2 mm (Figure 1H).
Discussion
The most important finding of the present study is that EES implantation in selected SVG lesions, most of which did not have in-stent restenosis, was associated with high rates of stent strut coverage in spite of high malapposition rates.
The SOS-Xience V is the first study of second-generation DESs in SVG lesions. In native coronary arteries, EESs have been associated with lower in-stent late loss10-14 and improved clinical outcomes15,16 compared to the paclitaxel-eluting stent (PES). First-generation DESs have been associated with promising angiographic and intravascular ultrasound imaging findings in SVGs.17,18 The present study shows that EES had low neointimal hyperplasia formation among patients of whom most did not have in-stent restenosis SVGs.17,18
 Uncovered stent struts are associated with increased risk of stent thrombosis.19,20 There are 4 studies9,21-23 that performed OCT evaluation for strut coverage and malapposition at 12 ± 1 months post stenting of native coronary arteries (the sirolimus-eluting stent was used in 3 studies and the PES was used in 1 study; Table 4). Our study shows lower rates of uncovered struts (3.8% vs 5.7%-10.5%) than in the published first-generation DES studies. However, higher rates of malapposition were observed (9.2% vs 0.9%-1.4%).
Uncovered stent struts are associated with increased risk of stent thrombosis.19,20 There are 4 studies9,21-23 that performed OCT evaluation for strut coverage and malapposition at 12 ± 1 months post stenting of native coronary arteries (the sirolimus-eluting stent was used in 3 studies and the PES was used in 1 study; Table 4). Our study shows lower rates of uncovered struts (3.8% vs 5.7%-10.5%) than in the published first-generation DES studies. However, higher rates of malapposition were observed (9.2% vs 0.9%-1.4%).
The high stent-strut coverage rates in our study are similar to those observed with EESs in native coronary arteries (Table 4): Inoue et al24 conducted an observational prospective study with 25 patients (5880 struts). At 8 months, the percentages of uncovered and malapposed struts were 1.64% and 0.52% respectively. Choi et al25 evaluated 40 patients (6782 struts) at 9 months, and found a percentage of strut uncoverage and malapposition of 4.4% and 0.4%, respectively. The high stent strut coverage rates observed with EESs may explain a possible association with lower stent thrombosis rates seen in some studies of native coronary arteries.15,16
The high rates of stent strut malapposition observed in the present study (9.2%) may be due to the large caliber and intragraft variability of luminal diameter of SVGs, although SVGs included in the present study (reference lumen diameter, 2.64 ± 0.80 mm) were smaller than SVGs included in previous studies (reference lumen diameter, 3.17 ± 0.75 mm in SOS3 vs 3.28 ± 0.57 mm in RRISC26). Moreover, SVG atheromas tend to have more foam cells and inflammatory cells27 and may be more likely to undergo positive remodeling.28 Although malapposition may predispose to stent thrombosis,29-31 many of the malapposed stent struts in our study were covered (205 of 241 malapposed struts; 85%) (Figure 1F and 1G), which may in part mitigate the increased stent thrombosis risk. The present study is too small to assess whether stent strut malapposition may translate to increased risk for late and very late stent thrombosis; however, the lack of any stent thrombosis during the 12 months of follow-up is reassuring.
Study limitations. Our study is limited by the small number of patients included. Although no significant differences were observed between patients who did and those who did not undergo OCT, given the small number of patients included, comparisons would be unlikely to show statistically significant differences, even when real differences that could affect OCT indices were present. Most patients were men, limiting extrapolation in women, although most patients undergoing SVG stenting in the overall US population are men.32 Given that OCT was not available for clinical use at the beginning of the SOS-Xience V study, and given the losses to angiographic follow-up, only a few patients underwent OCT evaluation, potentially introducing bias in the interpretation of the results. Moreover, OCT evaluation was not available immediately after stenting to determine whether malapposition was present at baseline or developed later. Only 1 patient with angiographic restenosis underwent OCT evaluation, which was due in part to the fact that 2 of the 6 restenoses were occlusive. Whether the OCT findings observed with EES can translate to improved clinical outcomes requires evaluation in ongoing prospective studies with a primary clinical endpoint, such as the Drug-Eluting Stents vs Bare-Metal Stents In Saphenous Vein Graft Angioplasty (DIVA, NCT01121224) trial.
Conclusion
In summary, OCT evaluation of EES in SVGs 12 months post implantation among 12 patients, most of whom did not have in-stent restenosis, demonstrates high rates of stent strut coverage, in spite of high rates of stent strut malapposition. Larger studies with a clinical primary endpoint and long-term follow-up are needed to assess the clinical implications of these findings.
References
- Brilakis ES, Lichtenwalter C, Abdel-karim A-rR, et al. Continued Benefit From Paclitaxel-Eluting Compared With Bare-Metal Stent Implantation in Saphenous Vein Graft Lesions During Long-Term Follow-Up of the SOS (Stenting of Saphenous Vein Grafts) Trial. J Am Coll Cardiol Intv 2011;4:176-182.
- Mehilli J, Pache J, Abdel-Wahab M, et al. Drug-eluting versus bare-metal stents in saphenous vein graft lesions (ISAR-CABG): a randomised controlled superiority trial. Lancet 2011;378:1071-1078.
- Brilakis ES, Lichtenwalter C, de Lemos JA, et al. A randomized controlled trial of a paclitaxel-eluting stent versus a similar bare-metal stent in saphenous vein graft lesions the SOS (Stenting of Saphenous Vein Grafts) trial. J Am Coll Cardiol 2009;53:919-928.
- Vermeersch P, Agostoni P, Verheye S, et al. Increased late mortality after sirolimus-eluting stents versus bare-metal stents in diseased saphenous vein grafts: results from the randomized DELAYED RRISC Trial. J Am Coll Cardiol 2007;50:261-267.
- Murata A, Wallace-Bradley D, Tellez A, et al. Accuracy of optical coherence tomography in the evaluation of neointimal coverage after stent implantation. JACC Cardiovasc Imaging 2010;3:76-84.
- Brilakis ES, Papayannis AC, Abdel-Karim ARR, et al. Prospective evaluation of the Xience V everolimus-eluting stent in saphenous vein graft atherosclerosis: the Xience V-SVG angiographic study. J Am Coll Cardiol. 2011;58:B59.
- Sawada T, Shite J, Negi N, et al. Factors that influence measurements and accurate evaluation of stent apposition by optical coherence tomography. Assessment using a phantom model. Circ J. 2009;73(10):1841-1847.
- Tanigawa J, Barlis P, Di Mario C. Intravascular optical coherence tomography: optimisation of image acquisition and quantitative assessment of stent strut apposition. EuroIntervention. 2007;3(1):128-136.
- Guagliumi G, Costa MA, Sirbu V, et al. Strut coverage and late malapposition with paclitaxel-eluting stents compared with bare metal stents in acute myocardial infarction: optical coherence tomography substudy of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial. Circulation. 2011;123(3):274-281.
- Grube E, Sonoda S, Ikeno F, et al. Six- and twelve-month results from first human experience using everolimus-eluting stents with bioabsorbable polymer. Circulation. 2004;109(18):2168-2171.
- Garg S, Serruys P, Onuma Y, et al. 3-year clinical follow-up of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with de novo coronary artery lesions: the SPIRIT II trial (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions). JACC Cardiovasc Interv. 2009;2(12):1190-1198.
- Stone GW, Midei M, Newman W, et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008;299(16):1903-1913.
- Serruys PW, Ong AT, Piek JJ, et al. A randomized comparison of a durable polymer everolimus-eluting stent with a bare metal coronary stent: the SPIRIT first trial. EuroIntervention. 2005;1(1):58-65.
- Serruys PW, Ruygrok P, Neuzner J, et al. A randomised comparison of an everolimus-eluting coronary stent with a paclitaxel-eluting coronary stent:the SPIRIT II trial. EuroIntervention. 2006;2(3):286-294.
- Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010;362(18):1663-1674.
- Kedhi E, Joesoef KS, McFadden E, et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375(9710):201-209.
- Brilakis ES, Saeed B, Banerjee S. Drug-eluting stents in saphenous vein graft interventions: a systematic review. EuroIntervention. 2010;5(6):722-730.
- Jeroudi O, Abdel-Karim AR, Michael TT, et al. Paclitaxel-eluting stents reduce neointimal hyperplasia compared to bare metal stent in saphenous vein grafts: results from the SOS (Stenting of Saphenous Vein Grafts) trial. EuroIntervention. 2011;7(8):948-954.
- Finn AV, Joner M, Nakazawa G, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115(18):2435-2441.
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48(1):193-202.
- Katoh H, Shite J, Shinke T, et al. Delayed neointimalization on sirolimus-eluting stents: 6-month and 12-month follow up by optical coherence tomography. Circ J. 2009;73(6):1033-1037.
- Yao ZH, Matsubara T, Inada T, Suzuki Y, Suzuki T. Neointimal coverage of sirolimus-eluting stents 6 months and 12 months after implantation: evaluation by optical coherence tomography. Chin Med J (Engl). 2008;121(6):503-507.
- Tian F, Chen YD, Sun ZJ, et al. Evaluation of neointimal coverage of overlapping sirolimus-eluting stents by optical coherence tomography. Chin Med J (Engl). 2009;122(6):670-674.
- Inoue T, Shite J, Yoon J, et al. Optical coherence evaluation for everolimus-eluting stents at 8 months after implantation (Cooperative Study with Korea). Heart. 2010;97(17):1379-1384.
- Choi HH, Kim JS, Yoon DH, et al. Favorable neointimal coverage in everolimus-eluting stent at 9 months after stent implantation: comparison with sirolimus-eluting stent using optical coherence tomography. Int J Cardiovasc Imaging. 2012;28(3):491-497.
- Vermeersch P, Agostoni P, Verheye S, et al. Randomized double-blind comparison of sirolimus-eluting stent versus bare-metal stent implantation in diseased saphenous vein grafts: six-month angiographic, intravascular ultrasound, and clinical follow-up of the RRISC trial. J Am Coll Cardiol. 2006;48(12):2423-2431.
- Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97(9):916-931.
- Hong M-K, Mintz GS, Hong MK, et al. Intravascular ultrasound assessment of the presence of vascular remodeling in diseased human saphenous vein bypass grafts. Am J Cardiol. 1999;84(9):992-998.
- Virmani R, Guagliumi G, Farb A, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109(6):701-705.
- Nakazawa G, Finn AV, Vorpahl M, Ladich ER, Kolodgie FD, Virmani R. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. J Am Coll Cardiol. 2011;57(4):390-398.
- Cook S, Wenaweser P, Togni M, et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation. 2007;115(18):2426-2434.
- Brilakis ES, Wang TY, Rao SV, et al. Frequency and predictors of drug-eluting stent use in saphenous vein bypass graft percutaneous coronary interventions: a report from the American College of Cardiology National Cardiovascular Data CathPCI Registry. JACC Cardiovasc Interv. 2010;3(10):1068-1073.
_______________________________________________
From the 1VA North Texas Healthcare System and 2University of Texas Southwestern Medical Center, Dallas, Texas.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Michael reports support from a T32HL007360 Cardiovascular Training Grant from the National Institutes of Health. Dr Addo is a member of Astra Zeneca’s speaker’s bureau. Dr Banerjee reports grants from the Medicines Company and Gilead; consultant positions with Medtronic and Covidien; ownership interests in HygeaTel and Mdcareglobal (spouse); and honoraria from Abbott (spouse). Dr Brilakis reports speaker honoraria from St Jude and Terumo; research support from Abbott Vascular; and employment with Medtronic (spouse). The other authors report no conflicts of interest regarding the content herein.
Manuscript submitted January 25, 2012, provisional acceptance given February 27, 2012, final version accepted March 23, 2012.
Address for correspondence: Emmanouil S. Brilakis, MD, PhD, Dallas VA Medical Center (111A), 4500 South Lancaster Road, Dallas, TX 75216. Email: esbrilakis@yahoo.com













