Neointimal Tissue Healing Patterns After Paclitaxel-Eluting Balloon Treatment of In-Stent Restenosis: Optical Coherence Tomography and Intravascular Ultrasound Insights
ABSTRACT: An 80-year-old patient presented with severe in-stent restenosis of an everolimus-eluting stent implanted in the left anterior descending coronary artery 3 years previously. We obtained good angiographic result after paclitaxel-eluting balloon dilation. However, on optical coherence tomography (OCT), multiple, angiographically silent, in-stent, and edge-related dissections were readily recognized. Intravascular ultrasound (IVUS) revealed residual neointima with minor disruptions. At 9-month follow-up, an excellent angiogiographic result was demonstrated with complete resolution of the stent-related dissections on OCT. IVUS and OCT confirmed complete neointimal healing with a larger lumen. This case illustrates the value of OCT and IVUS to provide unique insights on the pathophysiological mechanisms and healing patterns of paclitaxel-eluting balloon treatment of in-stent restenosis.
J INVASIVE CARDIOL 2012;24(10):E215-E218
Key words: In-stent restenosis, paclitaxel-eluting balloon, optical coherence tomography, intravascular ultrasound, coronary dissections, vascular healing
______________________________________________
Coronary artery dissections are frequently detected on angiography after balloon angioplasty.1 Coronary dissections provide an important mechanism for the acute lumen gain after balloon dilation.1 These dissections tend to have a good prognosis and heal at follow-up.1 Likewise, following stent implantation, residual coronary dissections may be identified at the stent edges.2-5 Most stent-related residual dissections also have a benign prognosis and tend to heal at long-term angiographic follow-up.2-5 Finally, dissections within the stent may also be identified in patients treated for in-stent restenosis (ISR). However, the exact mechanism of vessel wall healing leading to disappearance of dissections after interventions remains unknown.2-5 Intravascular ultrasound (IVUS), and more recently optical coherence tomography (OCT), provide unique tomographic insights of the vessel wall, thus facilitating a comprehensive anatomic assessment of the dissections induced by coronary interventions.5,6 In particular, OCT has an unprecedented axial resolution (150 μm) and, therefore, appears ideally suited to study coronary dissection healing patterns.6
The use of paclitaxel-eluting balloons (PEB) represents an attractive alternative in the treatment of ISR.7-10 In patients with bare-metal stent and drug-eluting stent ISR, PEBs provide superior long-term clinical and angiographic results as compared with conventional balloon angioplasty.7-10 Although the angiographic results of PEB in patients with ISR are largely satisfactory, scarce information exists on the underlying mechanisms of lumen gain and the healing patterns of this novel therapy. We describe a patient successfully treated with PEB for ISR in whom OCT unraveled the presence of angiographically silent intra-stent and stent-edge-related dissections. IVUS just disclosed minor irregularities in the residual neointima. At follow-up, both intracoronary diagnostic techniques demonstrated an adequate neointimal healing with a larger lumen, full stent coverage and complete resolution of the dissection images.
 Case Report. A 77-year-old male patient with hypertension, hyperlipidemia, and history of cigarette smoking was investigated for effort angina. Coronary angiography revealed 2-vessel disease with severe lesions in the proximal and mid left anterior descending coronary artery (LAD) and in the mid left circumflex coronary artery (LCx). Left ventricular function was normal with an ejection fraction of 75%. Two everolimus-eluting stents (Xience Prime, Abbott Vascular) (2.75 mm x 12 mm and 2.5 mm x 12 mm) were implanted in the proximal and mid LAD and another everolimus-eluting stent (2.5 mm x 23 mm) was deployed in the LCx, all with good angiographic results. Three years later the patient presented with recurrent effort angina. Coronary angiography disclosed a severe ISR of the proximal edge of the stent implanted in the proximal LAD (Figure 1A).
Case Report. A 77-year-old male patient with hypertension, hyperlipidemia, and history of cigarette smoking was investigated for effort angina. Coronary angiography revealed 2-vessel disease with severe lesions in the proximal and mid left anterior descending coronary artery (LAD) and in the mid left circumflex coronary artery (LCx). Left ventricular function was normal with an ejection fraction of 75%. Two everolimus-eluting stents (Xience Prime, Abbott Vascular) (2.75 mm x 12 mm and 2.5 mm x 12 mm) were implanted in the proximal and mid LAD and another everolimus-eluting stent (2.5 mm x 23 mm) was deployed in the LCx, all with good angiographic results. Three years later the patient presented with recurrent effort angina. Coronary angiography disclosed a severe ISR of the proximal edge of the stent implanted in the proximal LAD (Figure 1A).
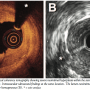 Angiographically, the narrowing that caused ISR encompassed the most proximal edge of the stent (4 mm) but also extended for 4 mm into the immediately adjacent proximal coronary vessel. OCT visualized a severe ISR with a minimal lumen area of 2.6 mm2 and a neointimal area of 6.1 mm2 obstructing 74% of the stent area (8.7 mm2). At the target lesion site in-stent neointimal volume was 28.7 mm3 (Figure 2). The lesion was predilated with a conventional balloon (2.5 mm x 10 mm, Fire Star, Cordis) at 22 bar and, subsequently, a PEB (3.0 mm x 10 mm, Sequent Please, B Braun) was used (60 seconds at 7 bar) to obtain a good angiographic result (Figure 1B). Post-intervention OCT demonstrated lumen improvement with a minimal lumen area of 4.9 mm2 and a residual neointimal area of 4.1 mm2 still obstructing 46% of stent area (9 mm2). Residual in-stent neointimal volume was 20.8 mm.3 The mean lumen area gain after the procedure was 0.35 ± 0.75 mm2 (Figure 3). In addition, several striking images of dissection within the stent and at the stent edges were visualized. IVUS findings were very similar although intra-stent and edge dissections were poorly recognized (Figure 3). Interestingly, all these findings were angiographically silent.
Angiographically, the narrowing that caused ISR encompassed the most proximal edge of the stent (4 mm) but also extended for 4 mm into the immediately adjacent proximal coronary vessel. OCT visualized a severe ISR with a minimal lumen area of 2.6 mm2 and a neointimal area of 6.1 mm2 obstructing 74% of the stent area (8.7 mm2). At the target lesion site in-stent neointimal volume was 28.7 mm3 (Figure 2). The lesion was predilated with a conventional balloon (2.5 mm x 10 mm, Fire Star, Cordis) at 22 bar and, subsequently, a PEB (3.0 mm x 10 mm, Sequent Please, B Braun) was used (60 seconds at 7 bar) to obtain a good angiographic result (Figure 1B). Post-intervention OCT demonstrated lumen improvement with a minimal lumen area of 4.9 mm2 and a residual neointimal area of 4.1 mm2 still obstructing 46% of stent area (9 mm2). Residual in-stent neointimal volume was 20.8 mm.3 The mean lumen area gain after the procedure was 0.35 ± 0.75 mm2 (Figure 3). In addition, several striking images of dissection within the stent and at the stent edges were visualized. IVUS findings were very similar although intra-stent and edge dissections were poorly recognized (Figure 3). Interestingly, all these findings were angiographically silent.
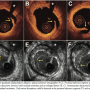 At the scheduled 9-month follow-up, an excellent angiographic result was found in the target stent on the proximal LAD (Figure 1C). IVUS and OCT demonstrated homogeneous mild intimal thickening along the entire stent. OCT (at exactly the same location) showed a minimal lumen area of 6.3 mm2 and residual neointimal area of 2.7 mm2, obstructing 30% of the stent area. In-stent neointimal volume was 15.9 mm.3 The final mean lumen area gain (from baseline) was 0.86 ± 1.02 mm2 (Figure 4). OCT also showed all stent
At the scheduled 9-month follow-up, an excellent angiographic result was found in the target stent on the proximal LAD (Figure 1C). IVUS and OCT demonstrated homogeneous mild intimal thickening along the entire stent. OCT (at exactly the same location) showed a minimal lumen area of 6.3 mm2 and residual neointimal area of 2.7 mm2, obstructing 30% of the stent area. In-stent neointimal volume was 15.9 mm.3 The final mean lumen area gain (from baseline) was 0.86 ± 1.02 mm2 (Figure 4). OCT also showed all stent  struts were nicely covered. IVUS findings were similar with a homogeneous neointima and a smooth lumen. No malapposed stent struts were visualized with either technique. Moreover, no trace of residual dissections (in-stent or edge-related) were visualized with these techniques (Figure 4). Interestingly, the treated segment (previously with moderate residual hyperplasia) showed mild homogeneous neointimal coverage, morphologically indistinguishable from that seen at segments of the stent not previously treated.
struts were nicely covered. IVUS findings were similar with a homogeneous neointima and a smooth lumen. No malapposed stent struts were visualized with either technique. Moreover, no trace of residual dissections (in-stent or edge-related) were visualized with these techniques (Figure 4). Interestingly, the treated segment (previously with moderate residual hyperplasia) showed mild homogeneous neointimal coverage, morphologically indistinguishable from that seen at segments of the stent not previously treated.
Discussion. Residual coronary dissections are frequently detected after coronary interventions.1-5 When these dissections are mild, associated with a large residual lumen and a normal flow, and remain stable after the procedure, they have an excellent prognosis.1-5 Actually, residual dissections frequently heal and disappear at long-term follow-up.1-5 IVUS and OCT are by far more sensitive than angiography to detect procedure-related coronary dissections and frequently detect minor dissections that are invisible on angiography.5,6 These tomographic techniques provide a comprehensive anatomic assessment of the stent-related dissections and are of great value to measure the residual coronary lumen and, therefore, may be used in the clinical decision making process during coronary interventions. Nevertheless, further studies are warranted to determine how this unique information should be used to guide and optimize the results of reinterventions in these challenging patients in order to improve clinical outcomes.
OCT has 10-fold higher resolution than IVUS and provides crisp images of unique quality even in tiny residual dissections.6 Likewise, OCT and IVUS are valuable to better understand the spontaneous, naturally occurring, healing process leading to the disappearance of these dissections at follow-up.5,6,11
To our knowledge this report represents the first description of the mechanisms underlying the acute procedural success of PEB in patients with ISR. Our findings provide unique pathophysiologic insights into the healing patterns of PEB-induced residual in-stent and edge-related dissections in these patients. Despite excellent angiographic results after PEB, significant residual neointima persists within the stent and the torn neointimal tissue generates multiple small in-stent residual dissections hanging freely into the lumen. In addition, dissection tears are detected at the stent edges. The latter appears to be associated with the residual plaque burden that remains at the stent edges in most patients. Of interest, all these findings were angiographically silent. Our findings also support the use of intracoronary imaging techniques to fully ascertain the healing patterns occurring after treatment of ISR with PEB. Complete healing of all previously detected residual dissections was demonstrated at follow-up. In fact, the residual neointima at the treated segment was even thinner at late follow-up and indistinguishable from that covering areas of the stent that never suffer from restenosis. Moreover, no uncovered stent struts were detected. All these findings suggest an adequate remodeling of the neointimal tissue and a complete healing process eventually leading to a larger lumen. However, additional imaging studies in patients with ISR would be required to confirm the possibility of distinct healing patterns after PEB as compared with conventional balloon angioplasty.
Our findings underscore the value of OCT and IVUS to unravel the precise mechanism of action of PEB in the treatment of patients with ISR and to elucidate the healing process of the residual neointimal tissue.
References
- Leimgruber PP, Roubin GS, Anderson HV, et al. Influence of intimal dissection on restenosis after successful coronary angioplasty. Circulation. 1985;72(3):530-535.
- Alfonso F, Hernandez R, Goicolea J, et al. Coronary stenting for acute coronary dissection after coronary angioplasty: implications of residual dissection. J Am Coll Cardiol. 1994;24(4):989-995.
- Biondi-Zoccai GG, Agostoni P, Sangiorgi GM, et al; for the Real-world Eluting-stent Comparative Italian retrosPective Evaluation Study Investigators. Incidence, predictors, and outcomes of coronary dissections left untreated after drug-eluting stent implantation. Eur Heart J. 2006;27(5):540-546.
- Alfonso F. Residual coronary dissections after drug-eluting stenting: the good, the bad, and the ugly. Eur Heart J. 2006;27(5):503-505.
- Hong MK, Park SW, Lee NH, et al. Long-term outcomes of minor dissection at the edge of stents detected with intravascular ultrasound. Am J Cardiol. 2000;86(7):791-795.
- Alfonso F, Canales E, Aleong G. Spontaneous coronary artery dissection: diagnosis by optical coherence tomography. Eur Heart J. 2009;30(3):385.
- Scheller B, Hehrlein C, Bocksch W, et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. 2006;355(20):2113-2124.
- Habara S, Mitsudo K, Kadota K, et al. Effectiveness of paclitaxel-eluting balloon catheter in patients with sirolimus-eluting stent restenosis. JACC Cardiovasc Interv. 2011;4(2):149-154.
- Unverdorben M, Vallbracht C, Cremers B, et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation. 2009;119(23):2986-2994.
- Rittger H, Brachmann J, Sinha AM, et al. A randomized, multicenter, single-blinded trial comparing paclitaxel-coated balloon angioplasty with plain balloon angioplasty in drug-eluting stent restenosis: the PEPCAD-DES Study. J Am Coll Cardiol. 2012;59(15):1377-1382.
- Alfonso F, Canales E, Dutary J, Cruz A. Coronary dissection healing patterns: from complete resolution to restenosis, insights from optical coherence tomography. EuroIntervention. 2011;7(2):270-273.
______________________________________________
From the Interventional Cardiology Department, Cardiovascular Institute, Clínico San Carlos University Hospital, IdISSC, Madrid, Spain.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted April 5, 2012, provisional acceptance given May 4, 2012, final version accepted May 16, 2012.
Address for correspondence: Fernando Alfonso, MD, PhD, FESC, Interventional Cardiology, Cardiovascular Institute, Clínico San Carlos University Hospital, IdISSC, Madrid, Spain. Email: falf@hotmail.com












