Multivessel Intervention and Placement of a Percutaneous Right Ventricular Assist Device in a Patient with Acute Myocardial Infarction Complicated by Cardiac Arrest
ABSTRACT: We present a case of a 66-year-old male who presented with ST elevation myocardial infarction and complicated by cardiac arrest. The patient underwent emergent multivessel revascularization. However, the patient developed right ventricular failure with persistent and recalcitrant hemodynamic instability. Placement of a TandemHeart™ right ventricular assist device™ (Cardiac Assist, Pittsburgh, Pennsylvania) was undertaken to allow for right ventricular recovery. The device was removed after several days and the patient has had no further events one year after arrest.
J INVASIVE CARDIOL 2011;23:248–251
Key words: hemodynamic support; right ventricular infarction; RVAD; TandemHeart
________________________________________
Acute left-sided ventricular failure after myocardial infarction (MI), with or without subsequent cardiac arrest, and need for support via left ventricular assist device placement has been well documented.1,2 Right ventricular infarction as a cause for cardiogenic shock occurs far less frequently, although the mortality associated with this condition is striking. Little is known about acute hemodynamic support of the right ventricle (RV) with mechanical assistance, and there are no documented cases of acute RV failure after MI requiring placement of a right ventricular assist device (RVAD).
Case Presentation
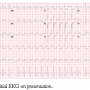 A 66-year-old male without a significant medical history presented to the emergency department with chest pain radiating to his shoulders. The initial electrocardiogram (Figure 1) revealed inferior ST elevations as well as ST depressions and tall R waves in V1-V3 consistent with acute inferoposterior MI, and the patient was transported emergently to the cardiac catheterization lab. Upon transport, the patient suffered arrest from pulseless ventricular tachycardia and immediate ACLS was initiated. The patient received a 200 joule shock with a biphasic defibrillator and returned to sinus rhythm. He was intubated and was noted to be profoundly hypotensive. Intravenous dopamine, dobutamine, and norepinephrine were initiated.
A 66-year-old male without a significant medical history presented to the emergency department with chest pain radiating to his shoulders. The initial electrocardiogram (Figure 1) revealed inferior ST elevations as well as ST depressions and tall R waves in V1-V3 consistent with acute inferoposterior MI, and the patient was transported emergently to the cardiac catheterization lab. Upon transport, the patient suffered arrest from pulseless ventricular tachycardia and immediate ACLS was initiated. The patient received a 200 joule shock with a biphasic defibrillator and returned to sinus rhythm. He was intubated and was noted to be profoundly hypotensive. Intravenous dopamine, dobutamine, and norepinephrine were initiated.
Upon arrival to the cardiac catheterization lab, bilateral femoral arterial access was obtained and an intra-aortic balloon pump (Maquet Cardiac Assist, Fairfield New Jersey) was placed and set at 1:1 with improvement in blood pressure from 58/50 mmHg to 101/74 mmHg. Despite this, the patient continued to have several episodes of sustained ventricular tachycardia requiring defibrillation and administration of amiodarone and lidocaine. Coronary angiography was performed and revealed a tubular 80% lesion in the proximal left anterior artery, a total occlusion of the proximal left circumflex artery, and a 95% proximal right coronary artery lesion with TIMI 2 flow.
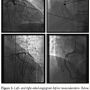 After initiation of anticoagulation with heparin and two boluses with subsequent infusion of Integrilin® (eptifibatide, Schering-Plough, Kenilworth, New Jersey), a 6 French (Fr) XB 4.5 guide catheter (Cordis Corporation, Miami Lakes, Florida) was seated in the ostium of the left main coronary artery. A 0.014-inch x 300 cm Asahi Prowater guidewire (Abbott Laboratories, Abbott Park, Illinois) was advanced distally in the left circumflex artery, where balloon angioplasty was performed with staged 2.0 mm x 12 mm and 3.0 mm x 12 mm Maverick balloons (Boston Scientific, Natick, Massachusetts). Next, overlapping 3.5 mm x 12 mm and 3.0 mm x 23 mm Liberté bare-metal stents (Boston Scientific) were deployed successfully with restoration of flow in the left circumflex. Given the patients poor hemodynamic status despite the use of multiple vasoactive agents, the decision was made to proceed with complete percutaneous revascularization. The Prowater guidewire was redirected into the left anterior descending (LAD) artery and into the large first diagonal branch. A second Prowater guidewire was placed into the distal LAD. After balloon angioplasty with a 2.5 mm x 20 mm Maverick balloon, a 3.0 mm x 23 mm Liberté bare-metal stent was deployed in the proximal LAD with resulting TIMI 3 flow. Next, a 6 Fr JR4 guide catheter (Cordis Corp.) was seated in the ostium of the right coronary artery. Through this, a 0.014-inch PT2 moderate support wire (Boston Scientific) was advanced. Balloon angioplasty was performed proximally with a 2.5 x 20 mm Voyager balloon (Abbott Vascular, Santa Clara, California) and stented with overlapping 3.0 mm x 23 mm and 3.0 mm x 12 mm Vision bare-metal stents (Abbott Vascular).
After initiation of anticoagulation with heparin and two boluses with subsequent infusion of Integrilin® (eptifibatide, Schering-Plough, Kenilworth, New Jersey), a 6 French (Fr) XB 4.5 guide catheter (Cordis Corporation, Miami Lakes, Florida) was seated in the ostium of the left main coronary artery. A 0.014-inch x 300 cm Asahi Prowater guidewire (Abbott Laboratories, Abbott Park, Illinois) was advanced distally in the left circumflex artery, where balloon angioplasty was performed with staged 2.0 mm x 12 mm and 3.0 mm x 12 mm Maverick balloons (Boston Scientific, Natick, Massachusetts). Next, overlapping 3.5 mm x 12 mm and 3.0 mm x 23 mm Liberté bare-metal stents (Boston Scientific) were deployed successfully with restoration of flow in the left circumflex. Given the patients poor hemodynamic status despite the use of multiple vasoactive agents, the decision was made to proceed with complete percutaneous revascularization. The Prowater guidewire was redirected into the left anterior descending (LAD) artery and into the large first diagonal branch. A second Prowater guidewire was placed into the distal LAD. After balloon angioplasty with a 2.5 mm x 20 mm Maverick balloon, a 3.0 mm x 23 mm Liberté bare-metal stent was deployed in the proximal LAD with resulting TIMI 3 flow. Next, a 6 Fr JR4 guide catheter (Cordis Corp.) was seated in the ostium of the right coronary artery. Through this, a 0.014-inch PT2 moderate support wire (Boston Scientific) was advanced. Balloon angioplasty was performed proximally with a 2.5 x 20 mm Voyager balloon (Abbott Vascular, Santa Clara, California) and stented with overlapping 3.0 mm x 23 mm and 3.0 mm x 12 mm Vision bare-metal stents (Abbott Vascular).
The patient was monitored in the intensive care unit, requiring norepinephrine 0.06 mcg/kg/min, vasopressin 0.04 units/min and dopamine 20mcg/kg/min infusions, as well as IABP support to maintain marginal blood pressure. Twenty-four hours after revascularization, right heart pressures remained significantly elevated (right atrium 8 mmHg, RV 36/7 mmHg, pulmonary artery 37/19 mmHg with a mean of 28 mmHg, and pulmonary capillary wedge pressure of 26 mmHg). Echocardiography revealed a left ventricular ejection fraction of 35% with inferoposterior hypokinesis and RV akinesis, suggesting the patient’s poor hemodynamic status was largely due to right ventricular failure given reasonable left ventricular contractile reserve. This was further supported by the recalcitrant hypotension despite fluid resuscitation, use of an IABP, and institution of multiple pressors. The physicians involved, including the cardiothoracic surgeons agreed that further mechanical support of the right ventricle could be helpful, if not life-saving. A HeartMate bi-ventricular assist device (Thoratec, Pleasanton, California) was considered but the patient was deemed too unstable to tolerate an operative procedure. As such, the decision was made to proceed to the cardiac catheterization lab for placement of a percutaneous right ventricular support device with TandemHeart™ (Cardiac Assist, Pittsburgh, Pennsylvania).
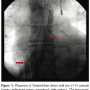 Bilateral femoral venous access was obtained, and 6 Fr sheaths were placed. Femoral venous angiography was performed, revealing near cessation of forward flow. A 6 Fr multipurpose catheter (Cordis Corp.) was advanced into the pulmonary artery through which a 0.035 inch x 260 cm Amplatz ExtraSupport wire (Cook Medical, Bloomington, Indiana) was placed. The 6 Fr sheath in the right femoral vein was removed and serial dilations were made to accommodate the 75 cm 21 Fr TandemHeart outflow cannula which was advanced from the right femoral vein into the pulmonary artery and clamped. A second 75 cm 21 Fr TandemHeart inflow cannula was placed after serial dilations from the left femoral vein and advanced to the right atrium and clamped. After confirming the position angiographically, the TandemHeart cannulae were connected to the intermediary motor via secure wet-to-wet connections and flow was initiated at 3 liters per minute.
Bilateral femoral venous access was obtained, and 6 Fr sheaths were placed. Femoral venous angiography was performed, revealing near cessation of forward flow. A 6 Fr multipurpose catheter (Cordis Corp.) was advanced into the pulmonary artery through which a 0.035 inch x 260 cm Amplatz ExtraSupport wire (Cook Medical, Bloomington, Indiana) was placed. The 6 Fr sheath in the right femoral vein was removed and serial dilations were made to accommodate the 75 cm 21 Fr TandemHeart outflow cannula which was advanced from the right femoral vein into the pulmonary artery and clamped. A second 75 cm 21 Fr TandemHeart inflow cannula was placed after serial dilations from the left femoral vein and advanced to the right atrium and clamped. After confirming the position angiographically, the TandemHeart cannulae were connected to the intermediary motor via secure wet-to-wet connections and flow was initiated at 3 liters per minute.
The patient was maintained on the RVAD and the IABP for bi-ventricular support and anticoagulated with heparin. Within 24 hours of RVAD placement the patient’s hemodynamics improved with reduction in heart rate from 87 bpm to 77 bpm, reduction in central venous pressure from 14 mmHg to 7 mmHg, and increase in blood pressure from 87/57 mmHg to 117/68 mmHg. Vasoactive agents were weaned two days after RVAD placement and the patient was successfully extubated. Given this clinical improvement and the ongoing risk of bleeding and infection with prolonged insertion, the RVAD cannulae and IABP were removed at bedside on day three with sustained hemodynamic stability. The patient was transferred to stepdown floor and subsequently discharged home on hospital day 7. Serial echocardiograms to evaluate his cardiac function one year after this event noted an ejection fraction of 61% with normal right ventricular performance.
Discussion
 This patient presented with acute inferoposterior MI with multivessel coronary disease and thrombotic occlusion of the left circumflex artery resulting in cardiogenic shock requiring multivessel PCI. Despite successful intervention, the patient’s hemodynamic status did not substantially improve secondary to RV failure. To our knowledge, we are the first to report on the use of TandemHeart ventricular support of the RV along with acute inferoposterior MI with RV failure in the setting of cardiogenic shock and successful resuscitation.
This patient presented with acute inferoposterior MI with multivessel coronary disease and thrombotic occlusion of the left circumflex artery resulting in cardiogenic shock requiring multivessel PCI. Despite successful intervention, the patient’s hemodynamic status did not substantially improve secondary to RV failure. To our knowledge, we are the first to report on the use of TandemHeart ventricular support of the RV along with acute inferoposterior MI with RV failure in the setting of cardiogenic shock and successful resuscitation.
The standard of care for primary PCI warrants intervention on the culprit lesion with staged intervention of non-culprit stenosis noted at the time of initial angiography. However, a meta-analysis of culprit vessel PCI versus multivessel PCI in ST-elevation MI reveals no detriment to the patient receiving complete revascularization immediately versus in a staged fashion. Patients who undergo multivessel PCI have a significant reduction in the rate of revascularizations, but no advantage in death and repeat MI.3 Six of the 10 studies evaluated in this meta-analysis included patients with cardiogenic shock (CS). This specific population was looked at in the Should We Emergently Revascularize Occluded Coronary for Cardiogenic Shock (SHOCK) trial, which randomized patients with CS to initial medical support including thrombolytics versus early revascularization. The study demonstrated significantly lower 6 month and 12 month mortality in the early revascularized arm,4,5 prompting the American Heart Association/American College of Cardiology to recommend that patients with CS receive early revascularization.6 There are limited data comparing coronary artery bypass surgery and multivessel PCI in patients with cardiogenic shock. Review of the literature reveals only observational studies on this matter, revealing the need for future research.7
Cardiogenic shock after myocardial infarction carries an in-hospital mortality of about 50–60%.4,8 Left ventricular failure accounts for 78% of cases of CS, but RV failure mortality accounts for 2.8% of CS mortality.9 Decreased RV function has been shown to be an independent predictor of total and cardiovascular mortality. Zornoff et al demonstrated that for each 5% decrease in fractional area change in the RV, there was a 16% increased odds of cardiovascular mortality.10 Ability to provide percutaneous hemodynamic support is available through intra-aortic balloon pump (IABP), Impella™ 2.5 (Abiomed, Danvers, Massachusetts) and the TandemHeart device. The use of Impella 2.5 has been demonstrated to significantly improve cardiac index in the setting of CS, far in excess of that provided by IABP,11 and has additionally been noted to increase capillary perfusion in the setting of acute anterior ST-elevation MI with the effect of reducing infarct size.12,13 Similarly, several studies have demonstrated a significant improvement in cardiac output, cardiac index, and MAP in cardiogenic shock patients with the use of TandemHeart.14–16 For instance, one study demonstrated a 1.2 L/min improvement in cardiac output and a 0.6 L/min/m2 improvement in cardiac index compared to baseline with the use of TandemHeart at maximum support, representing a significant 20% improvement in these hemodynamic parameters and an 18% improvement in MAP compared to support with an IABP.14 Clinically, this resulted in a significant increase in urine output per hour, as well as a reduction in lactate production. Meta-analysis of percutaneous support data demonstrates the use of the TandemHeart to be superior to IABP in providing higher cardiac index, higher mean arterial pressure and lower pulmonary capillary wedge pressure devices to provide left ventricular (LV) support during cardiogenic shock and recovery from myocardial insult, but no statistical difference in 30 day mortality compared to IABP.8
Despite the demonstrated utility of IABP, Impella and TandemHeart for LV failure, there are little data regarding the use of percutaneous support during right ventricular failure. There are case study reports that demonstrate the use of TandemHeart to provide right ventricular support for pulmonary hypertension and for cardiogenic shock after right coronary artery infarct,17–20 but none in the setting of cardiogenic shock with or without multivessel PCI such as this case.
Conclusion
To our knowledge, this is the first reported case of the use of percutaneous RV support with the use of TandemHeart after acute inferoposterior MI and resultant cardiogenic shock complicated by cardiac arrest from RV failure. In our experience, this method of support is a unique modality of circumventing the dire consequences of RV collapse from an ischemic insult, and further study is warranted.
Acknowledgements. We would like to acknowledge Janet Karol for all of her work in writing and proofreading this manuscript.
References
- Smalling RW, Sweeney M, Lachterman B, et al. Transvalvular left ventricular assistance in cardiogenic shock secondary to acute myocardial infarction. Evidence for recovery from near fatal myocardial stunning. J Am Coll Cardiol 1994;23:637–644.
- Thomas JL, Al-Ameri H, Economides C, et al. Use of a percutaneous left ventricular assist device for high-risk cardiac interventions and cardiogenic shock. J Invasive Cardiol 2010;22:360–364.
- Navarese EP, De Servi S, Buffon A, et al. Clinical impact of simultaneous complete revascularization vs. culprit only primary angioplasty in patients with st-elevation myocardial infarction and multivessel disease: A meta-analysis. J Thromb Thrombolysis 2011;31(2):217–225.
- Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock? N Engl J Med 1999;341:625–634.
- Hochman JS, Sleeper LA, White HD, et al. One-year survival following early revascularization for cardiogenic shock. JAMA 2001;285:190–192.
- Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol 2004;44:E1–E211.
- Metha R, Lopes R, Ballotta A, et al. Percutaneous coronary intervention or coronary artery bypass surgery for cardiogenic shock and multivessel coronary artery disease? Am Heart J 2010;159:141–147.
- Cheng JM, den Uil CA, Hoeks SE, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: A meta-analysis of controlled trials. Eur Heart J 2009;30:2102–2108.
- Hochman JS, Buller CE, Sleeper LA, et al. Cardiogenic shock complicating acute myocardial infarction--etiologies, management and outcome: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? J Am Coll Cardiol 2000;36:1063–1070.
- Zornoff LA, Skali H, Pfeffer MA, et al. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol 2002;39:1450–1455.
- Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008;52:1584–1588.
- Lam K, Sjauw KD, Henriques JP, et al. Improved microcirculation in patients with an acute ST-elevation myocardial infarction treated with the Impella LP2.5 percutaneous left ventricular assist device. Clin Res Cardiol 2009;98:311–318.
- Sjauw KD, Remmelink M, Baan J Jr, et al. Left ventricular unloading in acute ST-segment elevation myocardial infarction patients is safe and feasible and provides acute and sustained left ventricular recovery. J Am Coll Cardiol 2008;51:1044–1046.
- Burkhoff D, Cohen H, Brunckhorst C, O'Neill WW. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J 2006;152:469 e1–e8.
- Burkhoff D, O'Neill W, Brunckhorst C, et al. Feasibility study of the use of the TandemHeart percutaneous ventricular assist device for treatment of cardiogenic shock. Catheter Cardiovasc Interv 2006;68:211–217.
- Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2005;26:1276–1283.
- Atiemo AD, Conte JV, Heldman AW. Resuscitation and recovery from acute right ventricular failure using a percutaneous right ventricular assist device. Catheter Cardiovasc Interv 2006;68:78–82.
- Kiernan MS, Krishnamurthy B, Kapur NK. Percutaneous right ventricular assist via the internal jugular vein in cardiogenic shock complicating an acute inferior myocardial infarction. J Invasive Cardiol 2010;22:E23–E26.
- Prutkin JM, Strote JA, Stout KK. Percutaneous right ventricular assist device as support for cardiogenic shock due to right ventricular infarction. J Invasive Cardiol 2008;20:E215–E216.
- Rajdev S, Benza R, Misra V. Use of Tandem Heart as a temporary hemodynamic support option for severe pulmonary artery hypertension complicated by cardiogenic shock. J Invasive Cardiol 2007;19:E226–E229.
____________________________________
From the Section of Cardiology, Department of Medicine, The University of Chicago.
The authors report no financial relationships or conflicts of interest regarding the content herein.
Manuscript submitted February 3, 2011, provisional acceptance given March 14, 2011, final version accepted March 30, 2011.
Address for correspondence: Atman P. Shah, MD, 5841 South Maryland Avenue, MC 6080, Chicago, Illinois 60637. Email: ashah5@uchicago.edu












