A Modified Technique to Safely Close the Arterial Puncture Site After TAVI
Download a PDF of this article.
Abstract: Background. We describe a vascular closure technique, convenient in practice, that permits effective femoral artery closure after CoreValve (Medtronic) implantation during transcatheter aortic valve implantation (TAVI). Vascular complications of transfemoral access implantation have been associated with significantly increased patient morbidity and mortality, as well as with increased hospitalization, among patients undergoing TAVI. Technique. The crossover technique is performed while using the sheath dilatator in order to tightly grasp the crossover wire; a peripheral artery balloon is inserted in the iliac artery and inflated above the puncture site. The 18 Fr sheath is removed while hemostasis is achieved in that way. Minor vascular complications were observed in 10% (3 out of 30) of the patients treated with this vascular closure technique. No major vascular complications were observed. Conclusions. The described vascular closure maneuver is operator friendly, without demanding special skills and can be added to the therapeutic quiver for minimizing vascular complications after TAVI.
J INVASIVE CARDIOL 2013;25(1):45-47
Key words: vascular complications, TAVI
_____________________________________________
Transcatheter aortic valve implantation (TAVI) has been converted into a truly percutaneous procedure with the use of smaller sheaths (18 Fr) and new vascular closure devices. Nevertheless, it is still a technically demanding procedure, which sometimes may be accompanied by simple or even dreadful complications.1-3 Despite the less invasive approach, complications associated with vascular access are still reported with an incidence of 7%-15%.4,5 Vascular complications of transfemoral access implantation involve vessel dissection, perforation, rupture, pseudoaneurysm, and hematoma, and have been associated with significantly increased patient morbidity and mortality, as well as with increased hospitalization.6,7 The incidence of such complications underlines the need for the establishment of techniques that ensure a safe arterial puncture, bleeding interruption, and arterial closure at the puncture site. We describe a vascular closure technique, convenient in practice, that permits effective femoral artery closure after CoreValve implantation through an 18 Fr arterial sheath. It is a simplified approach, however, taking into consideration previously described techniques.8,9
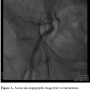 Standard procedure. Femoral artery cannulation for CoreValve (Medtronic) delivery is routinely performed utilizing the crossover technique. This includes a 5 Fr pigtail introduction contralaterally into the mid portion of the ipsilateral femoral artery in order to attain puncture of the arterial wall for the main access-site cannulation (Figure 1). Subsequently, the 10 Fr Prostar XL (Abbott Vascular) closure device is inserted and its two pairs of suture needles are brought out through the arteriotomy site and secured. Finally, the CoreValve Revalving System is introduced and implanted, as described elsewhere.10
Standard procedure. Femoral artery cannulation for CoreValve (Medtronic) delivery is routinely performed utilizing the crossover technique. This includes a 5 Fr pigtail introduction contralaterally into the mid portion of the ipsilateral femoral artery in order to attain puncture of the arterial wall for the main access-site cannulation (Figure 1). Subsequently, the 10 Fr Prostar XL (Abbott Vascular) closure device is inserted and its two pairs of suture needles are brought out through the arteriotomy site and secured. Finally, the CoreValve Revalving System is introduced and implanted, as described elsewhere.10
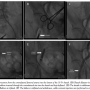 Novelty. After successful bioprosthesis deployment and removal of the introduction system, the 18 Fr arterial sheath is carefully withdrawn from the aorta until it reaches the level of the common iliac artery. Subsequently, while performing the crossover technique, with the help of the previously used 5 Fr pigtail, a 0.035˝ Terumo wire is advanced from the contralateral femoral artery into the lumen of the 18 Fr sheath until the bottom of its hub (Figure 2A). Afterward, the 18 Fr sheath dilatator is inserted and advanced in the sheath (Figure 2B). This stabilizes the Terumo wire, as it is tightly grasped between the sheath and dilatator walls. Thereafter, it can be used as a stable route for the over-the-wire delivery of the Monorail peripheral balloon (Boston Scientific).
Novelty. After successful bioprosthesis deployment and removal of the introduction system, the 18 Fr arterial sheath is carefully withdrawn from the aorta until it reaches the level of the common iliac artery. Subsequently, while performing the crossover technique, with the help of the previously used 5 Fr pigtail, a 0.035˝ Terumo wire is advanced from the contralateral femoral artery into the lumen of the 18 Fr sheath until the bottom of its hub (Figure 2A). Afterward, the 18 Fr sheath dilatator is inserted and advanced in the sheath (Figure 2B). This stabilizes the Terumo wire, as it is tightly grasped between the sheath and dilatator walls. Thereafter, it can be used as a stable route for the over-the-wire delivery of the Monorail peripheral balloon (Boston Scientific).
This double-lumen peripheral artery balloon (8 to 9 mm in diameter depending on common femoral or external iliac size) is inserted through the contralateral catheter into the 18 Fr sheath with an over-the-wire technique up to the iliac artery and kept deflated (Figure 2C). Thereafter, the wire is withdrawn and the balloon’s main lumen is connected to the manifold, allowing either arterial pressure monitoring at its peripheral edge or contrast injections. Afterward, the sheath is further withdrawn to a level just above the puncture site (Figure 2D). Subsequently, the Prostar-XL knots are pushed down to the femoral arterial wall. Then, the balloon is inflated, while hemostasis distally is ensured by pressure precipitation (Figure 2E).
The 18 Fr sheath is then safely removed, while the balloon is kept inflated for approximately 3 minutes and slightly stretched to the vessel wall.
Afterward, the balloon is deflated and slightly withdrawn, while contrast injections are performed through its central lumen to ensure the arterial sealing (Figure 2F). This maneuver not only serves the evaluation of potential leakage, but in case of inadequate hemostasis the balloon can be re-advanced at the bleeding site and inflated again. If no significant residual bleeding is observed, mechanical pressure is applied by the operator at the level of the puncture site. Finally, it is removed through the contralateral femoral artery.
Experience and 30-day outcomes. At our center, out of 115 patients treated with TAVI in the past 3 years, the last 30 aortic device implantations were conducted following the aforementioned technique during arterial closure. Minor vascular complication, as defined by the Valve Academic Research Consortium (VARC),11 was observed in 3 patients (10%) at the puncture site and treated successfully with balloon inflation. No major vascular complications were observed.
Discussion
We describe a simple vascular closure technique after TAVI with CoreValve prosthesis, which according to our experience can lead to a minimization of vascular complications. The precautionary balloon inflation above the puncture site promotes hemostasis and enables the appropriate vascular sealing, as suture stretching is performed under minor arterial pressure. During the procedure, pressure recording at the balloon edge represents a reliable and accurate method for ensuring vessel occlusion. Additionally, due to total vessel occlusion, the mechanical pressure applied at the site, if deemed necessary, is more effective for sealing, as there is no opposed hydrostatic tension.
Crossover technique from the contralateral femoral artery facilitates the delivery of endovascular devices for vascular complication management. Instead of delivering separate long sheaths and special back-up catheters, the required support for balloon delivery is acquired by inserting the 18 Fr sheath dilatator in the contralateral site, wedging the wire. In this way, the operator is able to cross over the balloon into the large sheath without worrying about catheter support.
Despite the preventive use of a peripheral vessel balloon in all patients, we believe that applying that technique for vessel closure could potentially be cost effective. The minimization of vascular complications such as rupture, perforation or hematoma renders the likelihood of using further rescue devices rather distant. Moreover, by wedging the wire with the sheath dilatator in the contralateral site, we avoid the use of further longer sheaths and extra back-up catheters.
In conclusion, we believe that the described vascular closure maneuver is operator friendly, without demanding special skills, and can fairly be added to the therapeutic quiver for minimizing vascular complications after TAVI. Our technique is in the same direction as similar effective vascular closure techniques proposed by other operators.8,9 However, further studies are needed in order to test and compare the short- and long-term complications and outcomes of the proposed techniques with more common practice methods.
References
- Hildick-Smith D, Redwood S, Mullen M, et al. Complications of transcatheter aortic valve implantation: avoidance and management. EuroIntervention. 2011;7(5):621-628.
- Vavouranakis M, Vrachatis DA, Toutouzas KP, et al. “Bail out” procedures for malpositioning of aortic valve prosthesis (CoreValve). Int J Cardiol. 2010;145(1):154-155.
- Vavuranakis M, Vrachatis D, Stefanadis C. CoreValve aortic bioprosthesis: repositioning techniques. JACC Cardiovasc Interv. 2010;3(5):565; author reply, 565-566.
- Hayashida K, Lefevre T, Chevalier B, et al. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc Interv. 2011;4(8):851-858.
- Gonze MD, Sternbergh WC 3rd, Salartash K, Money SR. Complications associated with percutaneous closure devices. Am J Surg. 1999;178(3):209-211.
- Van Mieghem NM, Nuis RJ, Piazza N, et al. Vascular complications with transcatheter aortic valve implantation using the 18 Fr Medtronic CoreValve System: the Rotterdam experience. EuroIntervention. 2010;5(6):673-679.
- Tchetche D, Dumonteil N, Sauguet A, et al. Thirty-day outcome and vascular complications after transarterial aortic valve implantation using both Edwards Sapien and Medtronic CoreValve bioprostheses in a mixed population. EuroIntervention. 2010;5(6):659-665.
- Genereux P, Kodali S, Leon MB, et al. Clinical outcomes using a new crossover balloon occlusion technique for percutaneous closure after transfemoral aortic valve implantation. JACC Cardiovasc Interv. 2011;4(8):861-867.
- Buchanan GL, Chieffo A, Montorfano M, et al. A ‘modified crossover technique’ for vascular access management in high-risk patients undergoing transfemoral transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2012 Apr 18. (Epub ahead of print).
- Vavuranakis M, Voudris V, Vrachatis DA, et al. Transcatheter aortic valve implantation, patient selection process and procedure: two centres’ experience of the intervention without general anaesthesia. Hellenic J Cardiol. 2010;51(6):492-500.
- Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57(3):253-269.
_____________________________________________
From the 1st Dept. of Cardiology, Hippokration Hospital, Medical School, National & Kapodistrian University of Athens, Athens, Greece.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted June 12, 2012 and final version accepted June 29, 2012.
Address for correspondence: Manolis Vavuranakis, Asst. Professor, 13 Astypaleas, Anoixi, Attiki-14569, Greece. Email: kalogerask@yahoo.gr and vavouran@otenet.gr












