Metal Fatigue in Myocardial Bridges: Stent Fracture Limits the Efficacy of Drug-Eluting Stents
ABSTRACT: Myocardial bridging (MB) is a common anatomical varient in which a segment of coronary artery takes an intramural path. Occasionally, it can result in symptomatic ischemia. We present four cases in which MB was treated with drug-eluting stents that subsequently fractured, leading to recurrent symptoms.
J INVASIVE CARDIOL 2011;23:E150–E152
_______________________________________
Myocardial bridging (MB) is a common anatomical variant in which a segment of coronary artery takes an intramural path. It is most often present in the left anterior descending artery (LAD). Though it is an incidental finding in most individuals, it can occasionally lead to symptomatic ischemia. The published experience of percutaneous coronary intervention (PCI) with stents is limited and there is no consensus on its long-term efficacy for the treatment of symptomatic patients.1,2 We present four cases in which symptomatic MB was treated with drug-eluting stents (DES) that subsequently fractured, resulting in recurrent symptoms.
 Case #1. A 61-year-old woman presented with angina. Angiography revealed MB of the mid-LAD (Figures 1A and 1B, Video 1). PCI was performed after medical therapy was ineffective. A 2.75 x 23 mm sirolimus-eluting Cypher stent (Cordis Corporation, Miami, Florida) was deployed without complications with complete relief of symptoms (Figure 1C, Video 2). Repeat coronary angiography was performed 4 months later for recurrence of angina and demonstrated in-stent restenosis (ISR) (Video 3) at the site of a partial stent fracture (Figures 1D and 1E, Video 4), which was treated with an everolimus-eluting 2.75 x 12 mm Xience stent (Abbott Vascular, Abbott Park, Illinois) with resolution of symptoms.
Case #1. A 61-year-old woman presented with angina. Angiography revealed MB of the mid-LAD (Figures 1A and 1B, Video 1). PCI was performed after medical therapy was ineffective. A 2.75 x 23 mm sirolimus-eluting Cypher stent (Cordis Corporation, Miami, Florida) was deployed without complications with complete relief of symptoms (Figure 1C, Video 2). Repeat coronary angiography was performed 4 months later for recurrence of angina and demonstrated in-stent restenosis (ISR) (Video 3) at the site of a partial stent fracture (Figures 1D and 1E, Video 4), which was treated with an everolimus-eluting 2.75 x 12 mm Xience stent (Abbott Vascular, Abbott Park, Illinois) with resolution of symptoms.
 Case #2. A 49-year-old man with a history of angina and MB presented with an acute anterior ST-elevation myocardial infarction. He also had a history of drug abuse, including amphetamines. Coronary angiography demonstrated mild-to-moderate atherosclerosis and MB of the mid-LAD, which contained thrombus (Figures 2A and 2B). There was normal flow in the vessel and PCI was not performed. Four months later, he suffered a non-ST elevation myocardial infarction and recurrent episodes of ventricular tachycardia. The MB in the mid-LAD was treated with a 3.5 x 33 mm Cypher stent (Figure 2C). Nine months later, he was admitted with recurrent angina and an angiogram demonstrated ISR due to stent fracture (Figures 2D and 2E, Video 5). Repeat PCI was not performed due to patient’s poor compliance with drug therapy and medical management was continued.
Case #2. A 49-year-old man with a history of angina and MB presented with an acute anterior ST-elevation myocardial infarction. He also had a history of drug abuse, including amphetamines. Coronary angiography demonstrated mild-to-moderate atherosclerosis and MB of the mid-LAD, which contained thrombus (Figures 2A and 2B). There was normal flow in the vessel and PCI was not performed. Four months later, he suffered a non-ST elevation myocardial infarction and recurrent episodes of ventricular tachycardia. The MB in the mid-LAD was treated with a 3.5 x 33 mm Cypher stent (Figure 2C). Nine months later, he was admitted with recurrent angina and an angiogram demonstrated ISR due to stent fracture (Figures 2D and 2E, Video 5). Repeat PCI was not performed due to patient’s poor compliance with drug therapy and medical management was continued.
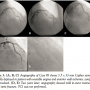 Case #3. An 88-year-old woman with unstable angina and evidence of anterior wall ischemia on a stress test underwent PCI to the mid-LAD using a 3.5 x 33 mm Cypher stent. The stent was deployed in a segment with atherosclerosis and severe MB (Figures 3A–3C) and was effective in treating the symptoms. The patient developed recurrent unstable angina 2 years later, and angiography demonstrated mild ISR at the site of a stent fracture (Figures 3D and 3E). PCI was not performed.
Case #3. An 88-year-old woman with unstable angina and evidence of anterior wall ischemia on a stress test underwent PCI to the mid-LAD using a 3.5 x 33 mm Cypher stent. The stent was deployed in a segment with atherosclerosis and severe MB (Figures 3A–3C) and was effective in treating the symptoms. The patient developed recurrent unstable angina 2 years later, and angiography demonstrated mild ISR at the site of a stent fracture (Figures 3D and 3E). PCI was not performed.
 Case #4. A 57-year-old male presented with limiting stable angina despite medical therapy. A coronary angiogram demonstrated mild atherosclerosis and severe MB in the distal-LAD (Figures 4A and 4B). Two Cypher stents (2.5 x 23 mm and 2.5 x 18 mm) were placed with overlap and resulted in complete relief of the angina (Figure 4C). The patient developed recurrent angina 6 months later and angiography confirmed severe focal ISR at the site of a minor stent fracture (Figures 4D and 4E). Coronary artery bypass grafting was performed using a left internal mammary artery graft to the LAD.
Case #4. A 57-year-old male presented with limiting stable angina despite medical therapy. A coronary angiogram demonstrated mild atherosclerosis and severe MB in the distal-LAD (Figures 4A and 4B). Two Cypher stents (2.5 x 23 mm and 2.5 x 18 mm) were placed with overlap and resulted in complete relief of the angina (Figure 4C). The patient developed recurrent angina 6 months later and angiography confirmed severe focal ISR at the site of a minor stent fracture (Figures 4D and 4E). Coronary artery bypass grafting was performed using a left internal mammary artery graft to the LAD.
Discussion. Our cases demonstrate that in some patients, MB with or without coexisting coronary artery disease may lead to stable angina or an acute coronary syndrome. Moreover, in the short-term, PCI appears to be effective in relieving angina in patients who remain symptomatic despite medical therapy. Prior case series of PCI for MB have predominantly reported experience with bare-metal stents in which the rate of target vessel revascularization was between 36–67%. External compression and neointimal proliferation were proposed as the potential mechanisms for the ISR, but stent fracture was not reported.1,3,4 DES have the potential for reducing the need for repeat revascularization; however, our experience highlights the fact that stent fracture may be an important mechanism leading to ISR and recurrent symptoms5 in this patient subset. Three of the four patients developed ISR within 6 months.
Stent fracture in DES was first reported in 2004 by Sianos and colleagues6 and its incidence has been estimated to be 1–3% in atherosclerotic lesions.7,8 In all four cases, the ISR was focal, consistent with previous reports.8 The fracture was partial, and its location in each of the patients was at the site of maximal compression by the bridge along the greater curvature of the LAD, suggesting that metal fatigue from repetitive mechanical stress on the stent struts during cardiac contractions was the likely mechanism. It has been suggested that long stents, overlap zones, and segments with marked angulations and hinge points also experience greater mechanical stress, factors that may have also contributed in our patients.
Our findings are supported by a recent study of 70 patients undergoing DES deployment for obstructive coronary artery disease in the proximal LAD who had angiographically silent (but intravascular ultrasound detectable) MB distal to the culprit lesion. In approximately one-third of patients, the stent extended beyond the obstructive lesion into the MB. These patients had significantly lower minimal stent area at the end of the procedure, and importantly, a higher rate of target lesion revascularization (24% versus 3%; p = 0.003) during a mean follow-up of 358 ± 252 days.9
Based on our experience and review of the published literature, we believe that stent fracture is an important mechanism for stent failure in MB. Thus, PCI should be reserved for severely symptomatic patients who have not responded to medical therapy. The patients must be informed that there is a significant possibility of requiring target vessel revascularization following PCI, even with the use of DES.
References
- Kursaklioglu H, Barcin C, Iyisoy A, et al. Angiographic restenosis after myocardial bridge stenting. Jpn Heart J 2004;45:581–589.
- Doshi AA, Orsini AR, Mazzaferri EL Jr, et al. Drug-eluting stent implantation for the treatment of symptomatic myocardial bridging is associated with favorable peri-procedural results and short-term outcomes. Int J Cardiol 2007;118:E87–E88.
- Kunamneni PB, Rajdev S, Krishnan P, et al. Outcome of intracoronary stenting after failed maximal medical therapy in patients with symptomatic myocardial bridge. Catheter Cardiovasc Interv 2008;71:185–190.
- Haager PK, Schwarz ER, vom Dahl J, et al. Long-term angiographic and clinical follow-up in patients with stent implantation for symptomatic myocardial bridging. Heart 2000;84:403–408.
- Tandar A, Whisenant BK, Michaels AD. Stent fracture following stenting of a myocardial bridge: Report of two cases. Catheter Cardiovasc Interv 2008;71:191–196.
- Sianos G, Hofma S, Ligthart JM, et al. Stent fracture and restenosis in the drug-eluting stent era. Catheter Cardiovasc Interv 2004;61:111–116.
- Doi H, Maehara A, Mintz GS, et al. Classification and potential mechanisms of intravascular ultrasound patterns of stent fracture. Am J Cardiol 2009;103:818–823.
- Aoki J, Nakazawa G, Tanabe K, et al. Incidence and clinical impact of coronary stent fracture after sirolimus-eluting stent implantation. Catheter Cardiovasc Interv 2007;69:380–386.
- Tsujita K, Maehara A, Mintz GS, et al. Impact of myocardial bridge on clinical outcome after coronary stent placement. Am J Cardiol 2009;103:1344–1348.
____________________________________
From the Division of Cardiovascular Diseases and Department of Internal Medicine, Mayo Clinic and Mayo Foundation, Rochester, Minnesota.
The authors report no conflicts of interest regarding the content herein.
Manuscript submitted September 29, 2010, provisional acceptance given November 1, 2010, final version accepted November 8, 2010.
Address for correspondence: Abhiram Prasad, MD, Mayo Clinic, 200 First Street SW, Rochester, MN 55905. Email: prasad.abhiram@mayo.edu














