Long-Term Outcomes of the Viabahn Stent in the Treatment of In-Stent Restenosis in the Superficial Femoral Artery
Abstract: There is no universally accepted method to treat in-stent restenosis (ISR) in the superficial femoral artery (SFA). It is hypothesized that using the Viabahn expandable polytetrafluoroethylene-covered stent to treat ISR may prevent tissue infiltration and intimal hyperplasia that leads to restenosis. Methods. We studied 22 patients (27 limbs) referred for treatment of severe ISR of the SFA. All patients were treated with the Viabahn stent implanted in the restenotic segments. We also analyzed several demographic, procedural, and laboratory parameters that could potentially be predictors of Viabahn restenosis. Results. Among patients treated, 63% had severe claudication and 37% had critical limb ischemia. Mean treated lesion length was 214.8 ± 87.2 mm, mean run-off score was 3.9 ± 2.8. Mean follow-up period was 21.8 ± 10.3 months. Ten patients (37%) developed Viabahn restenosis. The mean lesion length was 180.0 ± 107.9 mm in the restenosis group and 219.4 ± 78.9 mm in the no-restenosis group (P=.27). There was no significant difference between the two groups in the rest of demographic, procedural, and laboratory parameters. In 90%, restenosis occurred within the first 12 months and the remaining 10% occurred within 14 months. The mean time to restenosis was 6.2 ± 4.3 months. We observed no Viabahn ISR occurring after 14 month of follow-up. Conclusion. The Viabahn stent can be used to treat ISR in the SFA, with favorable results of 63% primary patency at up to 3 years of follow-up. Analysis of multiple factors showed no association with restenosis occurrence. If the Viabahn remained patent for 14 months, the likelihood of restenosis was low.
J INVASIVE CARDIOL 2013;25(12):670-674
Key words: in-stent restenosis, superficial femoral artery, Viabahn stent
___________________________
Due to the widespread use of stents in the treatment of occlusive atherosclerotic superficial femoral artery (SFA) disease, in-stent restenosis (ISR) has become an ever-increasing problem. One-year restenosis rates in the SFA after stenting range from 20%-50%,1-3 which is significantly higher compared to other vascular beds. This might be due to the unique anatomical location and forces imposed on the artery.4 With an incidence of 3%-10% in the general population and 15%-20% in persons greater than 70 years old,5-7 peripheral artery disease (PAD) is quite common; the lack of effective treatment of patients with PAD not only adds to patient suffering, but also raises concern for the tremendous healthcare costs involved.
To this day, there is no universally accepted method to effectively treat ISR in the SFA. Stent-grafts are, as the name suggests, a combination of stent with graft coating. Stent-grafts with expandable polytetrafluoroethylene (ePTFE) are promising tools for the treatment of ISR, because of the hypothesis that ePTFE-covered stents prevent tissue infiltration from intimal hyperplasia. The Gore Viabahn Endoprosthesis (W.L. Gore) has been used, with favorable results, to treat atherosclerotic lesions in the SFA and was shown to be superior to balloon angioplasty and comparable to bypass surgical revascularization procedure over a 12-month follow-up period.8 However, there are only few studies that address the efficacy of Viabahn for the treatment of ISR. Furthermore, there are even fewer studies that analyze the different patient characteristics that could potentially lead to failure of the Viabahn stent when it is used to treat ISR. This study aims to determine the long-term patency of the Viabahn stent used for treatment of SFA non-Viabahn ISR and whether there are any precipitating factors that could lead to its restenosis.
Methods
Using a retrospective case-control format, we evaluated 27 limbs (22 patients) referred to treatment for SFA ISR that were successfully treated with Viabahn stent (used at operator discretion) at the NYU Langone Medical Center catheterization lab from May 2008 to May 2011. Patients eligible for the study had prior nitinol bare-metal stents (non-Viabahn) placed in the SFA and subsequently became symptomatic with claudication or limb ischemia despite exercise and medical therapy and had severe occlusive ISR lesions on angiogram of the affected limb. These were then treated successfully with the Viabahn stent (used at operator discretion). Patients who had the Viabahn stent implanted for ISR treatment less than 6 months prior to enrollment were excluded. During follow-up, patency rates of the Viabahn stents after the procedure were evaluated clinically and using non-invasive imaging studies. Within the first year, all patients underwent clinical follow-up in conjunction with ankle-brachial index (ABI)/pulse-volume recording (PVR) study at least every 3 months, and then every 6 months thereafter (or sooner if symptoms occurred). Viabahn ISR was suspected if one or a combination of the following findings were observed: ABI drop of ≥0.15 from immediate postprocedure baseline and/or if the patient became symptomatic with claudication or limb ischemia. These findings led to initiation of further confirmatory testing using non-invasive imaging testing (arterial Duplex, computed tomography angiography) or invasive angiography. Diagnosis of ISR was considered to be established if the presence of obstructive ISR was demonstrated by the above-mentioned tests. By the study protocol, if the patient refused further confirmatory testing, he or she was considered to have ISR. Viabahn ISR was considered to be an endpoint for the study and these patients were no longer followed. Patients with Viabahn ISR were treated as per physician discretion.
We also analyzed several demographic characteristics, procedural characteristics, and laboratory parameters that have been reported in the literature as potential predictors of ISR, including age, weight, medications, creatinine levels, calculated glomerular filtration rate (GFR), white blood cell count (WBC), blood pressure, lipid level, random blood sugar, history of diabetes and chronic kidney disease, lesion length, and run-off score.9,10
Statistical analysis. P-values for continuous data were obtained using the two-tailed, independent t-test. For categorical data, the chi-squared test was used unless a category had less than 5 subjects, in which case the Fisher’s exact test was used. Statistical significance was determined if the P-value was <.05. Mean data are presented ± standard deviation. The Kaplan-Meier estimator (Figure 1) and statistical analysis were done with SPSS statistical software (IBM SPSS Statistics, version 21). Restenosis rates were calculated by dividing the number of restenosed limbs by the number of limbs that were evaluated during that time period. The same approach was applied to the subgroup analysis.
independent t-test. For categorical data, the chi-squared test was used unless a category had less than 5 subjects, in which case the Fisher’s exact test was used. Statistical significance was determined if the P-value was <.05. Mean data are presented ± standard deviation. The Kaplan-Meier estimator (Figure 1) and statistical analysis were done with SPSS statistical software (IBM SPSS Statistics, version 21). Restenosis rates were calculated by dividing the number of restenosed limbs by the number of limbs that were evaluated during that time period. The same approach was applied to the subgroup analysis.
Results
Baseline characteristics and lesion characteristics of all patients at the time of the Viabahn index procedure are shown in Tables 1 and 2, respectively. Out of 27 limbs treated, 15 (56%) received adjuvant atherectomy and balloon angioplasty (PTA) prior to Viabahn stent implantation, while 12 limbs (44%) were treated only with PTA and the Viabahn stent. The size of Viabahn used was 1 mm smaller in diameter compared to the size of the treated stent with ISR. All Viabahn stents were postdilated with 1:1 balloon-to-stent diameter ratio, with the balloon inflated above indicated nominal pressure for at least 2 minutes and until angiographically optimal stent apposition was achieved. Non-compliant balloons were used to achieve optimal result when the use of non-compliant balloon was insufficient. During the follow-up period, of the 27 limbs studied, 10 limbs restenosed (37%) and 17 remained patent (63%) for up to 40 months. The median and mean times to restenosis of Viabahn stent were 5.5 ± 4.3 months and 6.2 ± 4.3 months,
index procedure are shown in Tables 1 and 2, respectively. Out of 27 limbs treated, 15 (56%) received adjuvant atherectomy and balloon angioplasty (PTA) prior to Viabahn stent implantation, while 12 limbs (44%) were treated only with PTA and the Viabahn stent. The size of Viabahn used was 1 mm smaller in diameter compared to the size of the treated stent with ISR. All Viabahn stents were postdilated with 1:1 balloon-to-stent diameter ratio, with the balloon inflated above indicated nominal pressure for at least 2 minutes and until angiographically optimal stent apposition was achieved. Non-compliant balloons were used to achieve optimal result when the use of non-compliant balloon was insufficient. During the follow-up period, of the 27 limbs studied, 10 limbs restenosed (37%) and 17 remained patent (63%) for up to 40 months. The median and mean times to restenosis of Viabahn stent were 5.5 ± 4.3 months and 6.2 ± 4.3 months,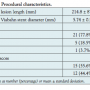 respectively. In 90% of the cases with restenosis, diagnosis occurred within the first 12 months of the procedure; restenosis occurred outside that time window in only 10% of cases. No restenosis was observed after 14 months (Figure 1). Several different criteria were examined to determine the potential cause of restenosis. Baseline characteristics of the groups with and without restenosis are listed in Table 3. No significant differences in baseline, procedural, and clinical characteristics were observed between groups with and without Viabahn restenosis. The treated length with Viabahn stents in the group without restenosis was numerically longer by 39 mm, with run-off
respectively. In 90% of the cases with restenosis, diagnosis occurred within the first 12 months of the procedure; restenosis occurred outside that time window in only 10% of cases. No restenosis was observed after 14 months (Figure 1). Several different criteria were examined to determine the potential cause of restenosis. Baseline characteristics of the groups with and without restenosis are listed in Table 3. No significant differences in baseline, procedural, and clinical characteristics were observed between groups with and without Viabahn restenosis. The treated length with Viabahn stents in the group without restenosis was numerically longer by 39 mm, with run-off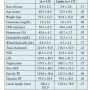 score numerically lower by 1.5, but neither reached statistical significance.
score numerically lower by 1.5, but neither reached statistical significance.
Clinical data that could potentially contribute to stent patency included gender, comorbidities (chronic kidney disease and diabetes), medications (cilostazol and statins), clinical presentation, and lesion severity (Rutherford class and TASC classification) are illustrated in Figure 2. We observed no significant differences between the groups.
Fifteen limbs (56%) received adjuvant atherectomy treatment along with PTA and Viabahn stent placement. Of the total number of limbs, 6 limbs (22.22%) received atherectomy and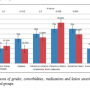 subsequently restenosed, while 9 limbs (33.33%) that received atherectomy remained patent. The comparison of these limbs with the limbs that did not receive atherectomy is illustrated in Figure 3. Of the 15 limbs that received atherectomy, 9 limbs (60%) underwent laser atherectomy, 5 limbs (33.33%) underwent Pathway atherectomy, and 1 limb (6.7%) underwent Silverhawk atherectomy.
subsequently restenosed, while 9 limbs (33.33%) that received atherectomy remained patent. The comparison of these limbs with the limbs that did not receive atherectomy is illustrated in Figure 3. Of the 15 limbs that received atherectomy, 9 limbs (60%) underwent laser atherectomy, 5 limbs (33.33%) underwent Pathway atherectomy, and 1 limb (6.7%) underwent Silverhawk atherectomy.
Discussion
The Viabahn stent-graft has been studied for its efficacy in the SFA versus bare nitinol stents and has been shown to have fewer stent fractures and overall better secondary patency, while primary patency was similar.11-19 It is hypothesized that use of stent-grafts for ISR treatment may prevent tissue infiltration and intimal hyperplasia, thus preventing future ISR. This hypothesis has been tested in several small studies. Shammeri et al studied 27 cases with femoro-popliteal ISR, with mean lesion length of 245 mm. In their study, the 1- and 3-year patency rates were 85.1% and 81.4%, respectively.20 On the other hand, Ansel et al studied 39 patients using the Viabahn stent with an average lesion length of 271 mm. Their 18-month follow-up patency rate was only 52%.21 In the SALVAGE study, the Viabahn stent was used to treat ISR along with excimer laser debulking, which demonstrated a 48% primary patency rate.22 Despite this low patency rate, patients experienced significant clinical improvements with higher ABIs, increased walking distances, higher speeds, and ability to climb stairs versus the non-Viabahn treatment group. Another study from Netherlands consisted of a comprehensive literature analysis on the application of Viabahn stent in the treatment of ISR in the SFA.23 Their results yielded a primary patency rate between 44% and 86%, with secondary patency rates between 58% and 93%. There was a clear improvement in patency versus angioplasty alone; however, there was a similar patency rate with prosthetic femoro-popliteal bypass up to 48 months.
femoro-popliteal ISR, with mean lesion length of 245 mm. In their study, the 1- and 3-year patency rates were 85.1% and 81.4%, respectively.20 On the other hand, Ansel et al studied 39 patients using the Viabahn stent with an average lesion length of 271 mm. Their 18-month follow-up patency rate was only 52%.21 In the SALVAGE study, the Viabahn stent was used to treat ISR along with excimer laser debulking, which demonstrated a 48% primary patency rate.22 Despite this low patency rate, patients experienced significant clinical improvements with higher ABIs, increased walking distances, higher speeds, and ability to climb stairs versus the non-Viabahn treatment group. Another study from Netherlands consisted of a comprehensive literature analysis on the application of Viabahn stent in the treatment of ISR in the SFA.23 Their results yielded a primary patency rate between 44% and 86%, with secondary patency rates between 58% and 93%. There was a clear improvement in patency versus angioplasty alone; however, there was a similar patency rate with prosthetic femoro-popliteal bypass up to 48 months.
In our study of 27 limbs treated for SFA ISR using the Viabahn stent, mean lesion length was 215 mm, and 3-year primary patency rate was 63%. The patency rate of 63% at 40-month follow-up that we observed was somewhat lower than reported previously by Al Shammeri et al, who reported 3-year patency of 81%; however, systematic non-invasive testing was not performed in that study, as was performed in the current report. The timing of restenosis in our study was notable, with no cases of restenosis observed beyond 14 months. Predictors of treatment failure with Viabahn stent-grafts have not been well described. In our study, we looked at the potential relationship of clinical and procedural characteristics described in the literature that might potentially effect the patency of SFA stents. Adjunctive atherectomy to debulk the lesion followed by balloon angioplasty prior to Viabahn implantation should theoretically lead to improvement of stent apposition and possibly to reduction of ISR. However, in our study, atherectomy had no effect on ISR rates. In our study, the group with no restenosis had numerically (219 mm vs 180 mm), but not statistically, longer lesions treated than the group with restenosis. This finding is consistent with previous studies demonstrating superiority of Viabahn stent patency when used for treatment of long de novo lesions in the SFA compared to nitinol bare-metal stents.24
It has been shown that run-off score has an impact on long-term outcomes after SFA endovascular intervention. At 5-year follow-up, the vessels with poor run-off had significantly higher rates of recurrent symptoms and restenosis.25 This was also proven in another study, which found run-off score >5 to significantly affect primary patency rates.26 In our study, a trend toward higher run-off score in patients with restenosis was notable as well, and highlights the importance of patency of the run-off vessels during SFA interventions.27 We observed no association between age, sex, blood pressure levels, lipid levels, GFR, creatinine, random blood glucose levels, different medication use, TASC category, presence of diabetes or kidney dysfunction, or occurrence of ISR in Viabahn. We could speculate that the small sample size of patients in our study explains why no statistically significant difference was demonstrated.
The literature reports on SFA ISR treatment consistently conclude that there is no universally accepted method to treat ISR, that larger studies need to be conducted to confirm the results, and that data are lacking on this topic. Along with these studies of the Viabahn stent-graft, a few other methods were examined to treat ISR that were found to provide suboptimal results; these include cutting-balloon angioplasty,28 atherectomy,29 drug-eluting balloons,30 cryoplasty,31 and repeat PTA and stenting with bare nitinol stents.32 In Germany, Zeller et al are performing the RELINE (The GORE VIABAHN Endoprosthesis with PROPATEN Bioactive Surface versus Plain Old Balloon Angioplasty (POBA) for the Treatment of Superficial Femoral Artery In-Stent Restenosis) trial, which is a multicenter (centers in Belgium and Germany), randomized clinical study determining the efficacy of the Viabahn stent versus bare nitinol stents in the treatment of ISR. As of now, it is the first trial of its kind to study Viabahn versus bare stents in a multicentered, randomized, clinical trial fashion. New (but not sufficiently studied) modalities of ISR treatment, such as drug-eluting balloons and stents, are currently available. Whether any of these approaches alone or in conjunction with other methods of treatment will prove to be superior and safer than any other methods remains to be seen. More data addressing the effectiveness of Viabahn stent-graft implantation for the treatment SFA ISR, as well as predictors of Viabahn restenosis, are required.
Our data did not reveal any particular contributing factor that led to restenosis of the Viabahn stent; however, there are some trends worth noting. For example, if the Viabahn stent remained patent for more than 14 months, then the chance of restenosis remained low. The median time to restenosis of Viabahn stent was 5.5 ± 4.3 months and no restenosis was observed after 14 months, as shown in Figure 1. If confirmed in larger studies (such as RELINE), this may have significant cost-saving implications, with no need for imaging follow-up of the implanted stent.
Study limitations. The limitations of a retrospective case control study are applicable to the current report. In addition, a larger sample size with longer follow-up might have yielded clinical, lesion, or procedural characteristics predictive of restenosis of Viabahn stents.
Conclusion
Viabahn stent-graft implantation appears to be an acceptable and safe treatment for SFA ISR. In this single-center study, we observed a 63% primary patency rate at 40-month follow-up exam. While we could not identify significant clinical, angiographic, or lesion-specific predictors of Viabahn graft restenosis or failure, ensuring the adequacy of the tibial run-off vessels may help guide the decision of whether to implant Viabahn stent-grafts in a given patient. Viabahn stents can be used to treat ISR in the SFA with favorable results of 63% primary patency rates at up to 3 years of follow-up. Analysis of multiple demographic factors, lesion characteristics, co-morbidities, and inflammatory markers showed no association with restenosis occurrence. If Viabahn stents remained patent for 14 months, the likelihood of restenosis was very low.
References
- Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354(18):1879-1888.
- Schillinger M, Sabeti S, Dick P, et al. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation. 2007;115(21):2745-2749.
- Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv. 2010;3(3):267-276.
- Zocholl G, Zapf S, Schild H, Thelen M. [Functional angiography of the arteries near the knee joint: consequences for stent implantation?]. Rofo. 1990;153(6):658-662.
- Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71(3):510-551.
- Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. The San Luis Valley Diabetes Study. Circulation. 1995;91(5):1472-1479.
- Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110(6):738-743.
- Saxon RR, Dake MD, Volgelzang RL, Katzen BT, Becker GJ. Randomized, multicenter study comparing expanded polytetrafluoroethylene-covered endoprosthesis placement with percutaneous transluminal angioplasty in the treatment of superficial femoral artery occlusive disease. J Vasc Interv Radiol. 2008;19(6):823-832.
- Razzouk L, Aggarwal S, Gorgani F, Babaev A. In-stent restenosis in the superficial femoral artery. Ann Vasc Surg. 2013;27(4):510-524.
- Biancari F, Albäck A, Ihlberg L, Kantonen I, Luther M, Lepäntalo M. Angiographic runoff score as a predictor of outcome following femorocrural bypass surgery. Eur J Vasc Endovasc Surg. 1999;17(6):480-485.
- Ansel GM, Lumsden AB. Evolving modalities for femoropopliteal interventions. J Endovasc Ther. 2009;16(Suppl II):1182-1197.
- Bauermeister G. Endovascular stent-grafting in the treatment of superficial femoral artery occlusive disease. J Endovasc Ther. 2001;8(3):315-320.
- Jahnke T, Andresen R, Muller-Hulsbeck S, et al. Hemobahn stent-grafts for treatment of femoropopliteal arterial obstructions: midterm results of a prospective trial. J Vasc Intervent Radiol. 2003;14(1):41-51.
- Lammer J, Dake MD, Bleyn J, et al. Peripheral arterial obstruction: prospective study of treatment with a transluminally placed self-expanding stent-graft. International Trial Study Group. Radiology. 2000;217(1):95-104.
- Railo M, Roth WD, Edgren J, et al. Preliminary results with endoluminal femoropopliteal thrupass. Ann Chir Gynaecol. 2001;90(1):15-18.
- Saxon RR, Coffman JM, Gooding JM, et al. Stent-graft use in the femoral and popliteal arteries. Tech Intervent Vasc Radiol. 2004;7(1):6-18.
- Turicchia GU, Cevolani M, Altini R, et al. Mid-term results in PTFE endograft treatment of femoropopliteal occlusive disease. Osp Ital Chir. 2003;9:93-96.
- Ansel GM, Geraghty PJ, Mewissen M, Jaff MR. 3-year VIBRANT results for bare stents and stent grafts in long SFA disease. Unpublished raw data.
- Geraghty PJ. Covered stenting of the superficial femoral artery using the viabahn stent-graft. Perspect Vasc Surg Endovasc Ther. 2006;18(1):39-45.
- Shammeri OA, Bitar F, Ghitelman J, Soukas PA. Viabahn for femoropopliteal in-stent restenosis. Ann Saudi Med. 2012;32(6):575-582.
- Ansel GM, Lumsden AB. Evolving modalities for femoropopliteal interventions. J Endovasc Ther. 2009;16(2 Suppl 2):II82-II97.
- Laird JR, Yeo KK, Rocha-Singh K et al. Excimer laser with adjunctive balloon angioplasty and heparin coated self-expanding stent grafts for the treatment of femoropopliteal artery in-stent restenosis: Twelve-month results from the SALVAGE study. Catheter Cardiovasc Interv. 2012;80(5):852-859.
- Doomernik DE, Golchehr B, Lensvelt MM, Reijnen MM. The role of superficial femoral artery endoluminal bypass in long de novo lesions and in-stent restenosis. J Cardiovasc Surg (Torino). 2012;53(4):447-457.
- Saxon RR, Coffman JM, Gooding JM, Ponec DJ. Long-term patency and clinical outcomes of the Viabahn stent-graft for femoropopliteal artery obstructions. J Vasc Interv Radiol. 2007;18(11):1341-1350.
- Davies MG, Saad WE, Peden EK, Mohiuddin IT, Naoum JJ, Lumsden AB. Percutaneous Superficial Femoral Artery Interventions for Claudication—Does Runoff Matter? Ann Vasc Surg. 2008;22(6):790-798.
- Ihnat DM, Duong ST, Taylor ZC, et al. Contemporary outcomes after superficial femoral artery angioplasty and stenting: the influence of TASC classification and runoff score. J Vasc Surg. 2008;47(5):967-974.
- Davies MG, Saad WE, Peden EK, Mohiuddin IT, Naoum JJ, Lumsden AB. Impact of runoff on superficial femoral artery endoluminal interventions for rest pain and tissue loss. J Vasc Surg. 2008;48(3):619-626.
- Dick P, Sabeti S, Mlekusch W et al. Conventional balloon angioplasty versus peripheral cutting balloon angioplasty for treatment of femoropopliteal artery in-stent restenosis: initial experience. Radiology. 2008;248(1):297-302.
- Zeller T, Rastan A, Sixt S et al. Long-term results after directional atherectomy of femoropopliteal lesions. J Am Coll Cardiol. 2006;48(8):1573-1578.
- Dake MD, Ansel GM, Jaff MR et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: Twelve month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4(5):495-504.
- Shin SH, Baril D, Chaer R, Makaroun M, Rhee R, Marone L. Cryoplasty offers no advantage over standard balloon angioplasty for the treatment of in-stent restenosis. J Vasc Surg. 2010;52(3):803-804.
- Trani C, Burzotta F, Tommasino A, Giammarinaro M. Transradial approach to treat superficial femoral artery in-stent restenosis. Catheter Cardiovasc Interv. 2009;74(3):494-498.
_________________________________
From the New York University Langone Medical Center Cardiac and Vascular Institute, Cardiac Catheterization Laboratory, New York, New York.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Babaev reports personal fees from Medtronic, Abbott, CSI, and Cook, outside the submitted work. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted May 20, 2013, provisional acceptance given July 19, 2013, final version accepted October 17, 2013.
Address for correspondence: Anvar Babaev, MD, PhD, 550 1st Ave, HCC 14 New York, NY 10016. Email: anvar.babaev@nyumc.org












