Investigation of Computed-Tomography Based Predictors of Acute Stroke Related to Transcatheter Aortic Valve Replacement: Aortic Wall Plaque Thickness Might be a Predictive Parameter of Stroke
Abstract: Objectives. Little information is available on computed tomography (CT)-based predictors of stroke related to transcatheter aortic valve replacement (TAVR). The objective of this study was to determine whether anatomical features of the aortic valve and aorta visualized by CT are predictive parameters of stroke. Methods. The study included 1270 patients who underwent preprocedural contrast-enhanced CT assessment and TAVR for severe aortic valve stenosis. Twenty-six patients (2.5%) who developed acute strokes that occurred within 48 hours after TAVR and 104 matched patients without strokes were identified, using 1:4 propensity-score matching. The degree of hypoattenuation in the aortic valve leaflets, calcium volume of the aortic valve, and plaque thickness in the aortic wall (the ascending aorta, aortic arch, and descending thoracic aorta) were assessed. Results. There were no differences between the two groups in the degree of hypoattenuation in the aortic valve leaflets and calcium volume of the aortic valve. The plaque thickness of the aortic arch and descending aorta were greater in the stroke group than in the non-stroke group: aortic arch, 2.4 mm (IQR, 1.3-2.8 mm) vs 1.8 mm (IQR, 1.4-2.2 mm), respectively (P<.01); and descending aorta, 2.9 mm (IQR, 2.1-4.2 mm) vs 2.8 mm (IQR, 2.1-3.6 mm); respectively (P=.049). Conclusion. Aortic wall plaque thickness measured by contrast-enhanced CT might be a predictive parameter of strokes that occur within 48 hours after TAVR.
Key words: aortic stenosis, computed tomography, stroke, plaque
Transcatheter aortic valve replacement (TAVR) is an alternative treatment to surgical aortic valve replacement in patients with severe, symptomatic aortic stenosis deemed to be at intermediate or high risk for surgery.1-7 Although the clinical outcomes of TAVR have improved considerably over time, stroke remains a concerning complication.8-16
The incidence of stroke at 30 days after TAVR ranges from 1.4%-6.8%.13-15,17-19 Strokes after TAVR, which do not include transient ischemic attacks, are associated with a 3-fold increase in 1-year mortality.14,15,20 Furthermore, asymptomatic strokes related to TAVR are more frequently observed. Ischemic strokes were detected by diffusion-weighted magnetic resonance (MRI) imaging in 68%-100% of patients undergoing TAVR.16,21-25
Elucidation of the predictors of stroke related to TAVR is important to further improve clinical outcomes. However, clinical studies to date have been disparate, reporting various predictors.20,26 Moreover, despite the widespread use of contrast-enhanced computed tomography (CT) in routine planning for TAVR to assess aortic annular dimensions, aortic root, and peripheral access vessels, no consensus on CT-based predictors of strokes exists.20,27,28
To investigate CT-based predictors of stroke related to TAVR, we focused on two anatomical elements, namely, aortic valve leaflets and aortic wall plaque. Aortic valve leaflets are a potential source of strokes because of the microembolization that can occur during the positioning and implantation of a transcatheter heart valve (THV).29,30 Aortic wall plaque may also serve as a potential site of embolic debris during delivery of the THV device via the transfemoral approach.24,29,31,32 This study aimed to determine whether these anatomical features detected by contrast-enhanced CT were predictive of periprocedural ischemic stroke related to TAVR.
Methods
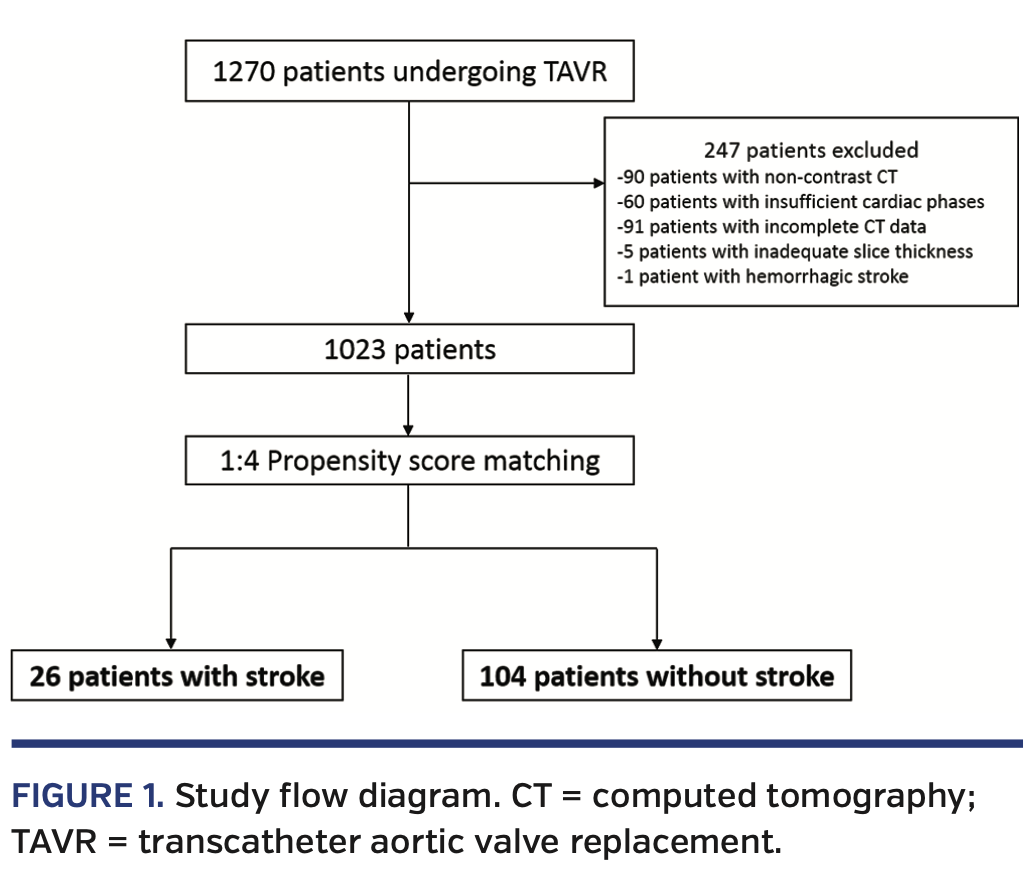
Study population. The study included 1270 consecutive patients who underwent preprocedural contrast-enhanced CT assessment and TAVR for severe aortic valve stenosis via the transfemoral approach at Cedars-Sinai Medical Center in Los Angeles, California between April 2012 and December 2016. We excluded patients with non-contrast CT (n = 90), insufficient cardiac phases (n = 60), incomplete CT data (n = 91), inadequate slice thickness (n = 5), and hemorrhagic stroke (n = 1). Of the remaining 1023 patients, we identified 26 patients (3.1%) with periprocedural ischemic strokes. Using 1:4 propensity-score matching, we matched 104 patients without stroke to identify a cohort of patients with similar baseline characteristics (Figure 1). TAVR was undertaken following consensus from a dedicated multidisciplinary heart team, including experienced clinical and interventional cardiologists and cardiovascular surgeons. The study complied with the Declaration of Helsinki. A locally appointed ethics committee approved the research protocol. Informed consent was obtained from all subjects.
Periprocedural stroke. Periprocedural stroke was defined as an acute cerebrovascular infarction causing focal or global neurologic dysfunction that occurred within 48 hours following TAVR and lasted for >24 hours. Neuroimaging evidence of infarction was required to confirm the diagnosis if neurologic dysfunction lasted for <24 hours. Of the 26 patients included for analysis, a brain MRI was conducted in 22 patients, a brain CT angiogram was performed in 3 patients, and no imaging was performed in 1 patient. The diagnosis of stroke was confirmed by an experienced neurologist in all cases.
TAVR procedure. All patients were pretreated with aspirin (100 mg) and clopidogrel (75 mg) prior to the procedure. Anticoagulant therapy including warfarin or dabigatran was discontinued 2-5 days before the procedure. If there was another indication for anticoagulant treatment, heparin bridging was performed. About 80–100 U/kg of heparin was intravenously administered to maintain an activating clotting time of >250 seconds during the procedure. The access site was determined by the multidisciplinary cardiovascular team. The decision regarding the type and size of the THV was at the primary operator’s discretion, using multidetector CT (MDCT) and/or three-dimensional transesophageal echocardiography (3D-TEE) during TAVR. The THV type implanted was either a balloon-expandable device or a self-expandable device. Balloon-expandable devices included the Sapien, the Sapien XT, and the Sapien 3 (Edwards Lifesciences). Self-expandable devices included the CoreValve and the Evolut R (Medtronic).
MDCT protocol and measurements. MDCT with electrocardiographic (ECG) gating was performed prior to TAVR with a Siemens Somatom Cardiac 64 or Siemens Somatom Flash scanner (Siemens Medical Solutions) using a collimation of 0.6 mm at a fixed pitch of 0.2 with an injection of 50-110 mL of iopamidol (Isovue-370; Bracco Imaging). A dedicated protocol was formulated with 120 kV, and the tube current was modified according to the patient’s size. Image acquisition was mainly performed with retrospective ECG gating. Digital Imaging and Communications in Medicine (DICOM) data were analyzed by a dedicated advanced imaging core laboratory, using 3-mensio Valves software, version 9.0 (Pie Medical Imaging).
MDCT image analysis. For annular and aortic valve complex (AVC) dimensions, curved multiplanar reconstruction analyses were performed using the 3-mensio Valves software that was specifically customized for valve analysis. A line was generated through the center-point of the proximal ascending aorta, aortic valve, annulus, and left ventricular outflow tract (LVOT).33 The basal annular plane was defined as the plane perpendicular to the curved multiplanar reconstruction line touching the nadir of the three leaflets.
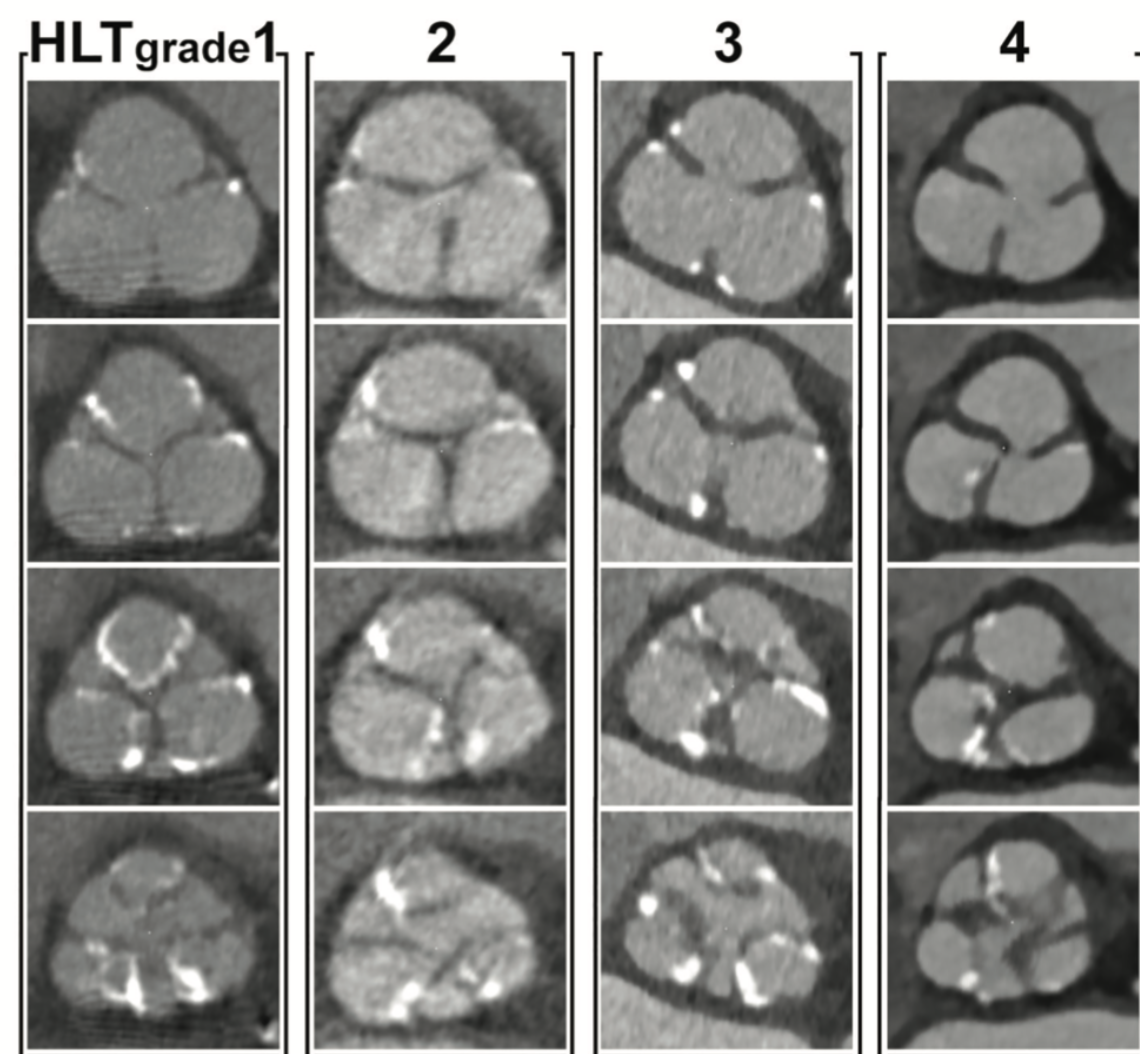
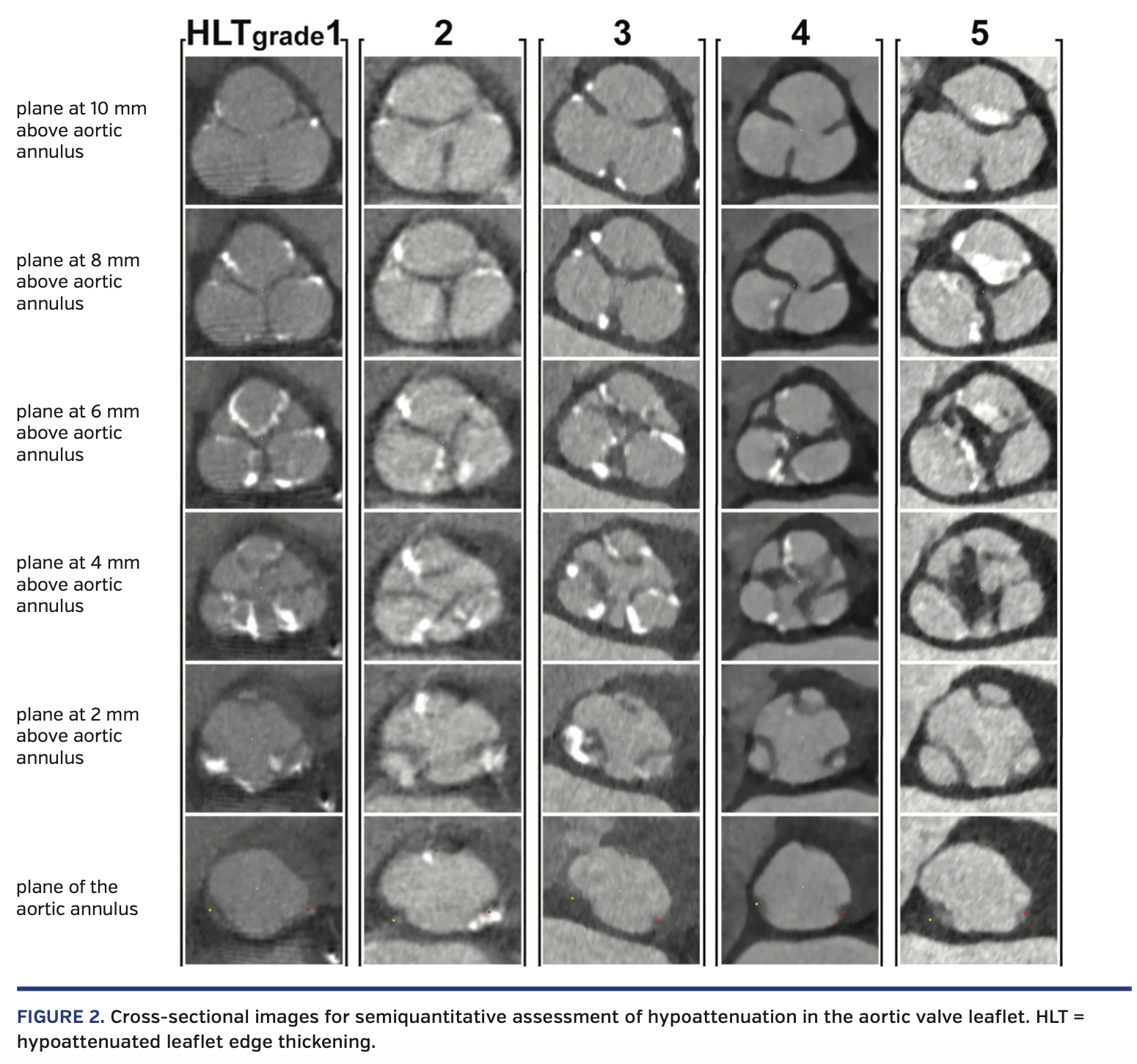
To evaluate the anatomical features of the aortic valve by contrast-enhanced CT, we performed a semiquantitative assessment of the aortic valve leaflet hypoattenuation and quantification of the calcium volume of the aortic valve complex. The aortic valve leaflet was visually evaluated from the basal annulus plane to the level of the commissures of the aortic cusps. To assess the degree of hypoattenuation in the aortic valve leaflet, hypoattenuated leaflet edge thickening (HLT) of the aortic valve was graded as an integer from one to five based on the examples in Figure 2. No hypoattenuated thickening of the aortic leaflet was classified as HLT grade 1; mildly thickened aortic leaflet HLT as grade 2; moderately thickened HLT as grade 3; severely thickened HLT as grade 4; and extremely thickened HLT as grade 5 (Figure 2). This assessment was performed by observers in the CT core laboratory. The observers were blinded to patient characteristics. Intraobserver and interobserver reproducibility of this measurements were satisfactory (intraclass correlation coefficient 1,1 = 0.82 [P<.001]; intraclass correlation coefficient 1,2 = 0.90 [P<.001]; interobserver = 0.76 [P<.001]. To increase the reproducibility of the results of this study, this measurement was performed twice, and the average value of the two measurements was used to define the HLT grade.
Contrast-enhanced CT was also used for localization of aortic-valvular complex calcium. Given the lack of consensus regarding the optimal threshold for detection of calcium predictive of strokes, four thresholds were used (450 Hounsfield Units [HU)], 550 HU, 650 HU, 850 HU) to assess the volume of calcium in the region of interest.34,35 The aortic-valvular complex was segmented into the following: leaflet (from 2 mm superior to the annular plane to the superior edge of the leaflet); annulus (from 2 mm inferior to the annular plane to 3 mm superior to the annular plane); LVOT (from 5 mm inferior to the annular plane to the annular plane); leaflet + annulus (from 2 mm inferior to the annular plane to the superior edge of the leaflet); and total (from 5 mm inferior to the annular plane to the superior edge of the leaflet)34 (Figure 3). The volume of calcification (Ca) was recorded for each of the five regions.
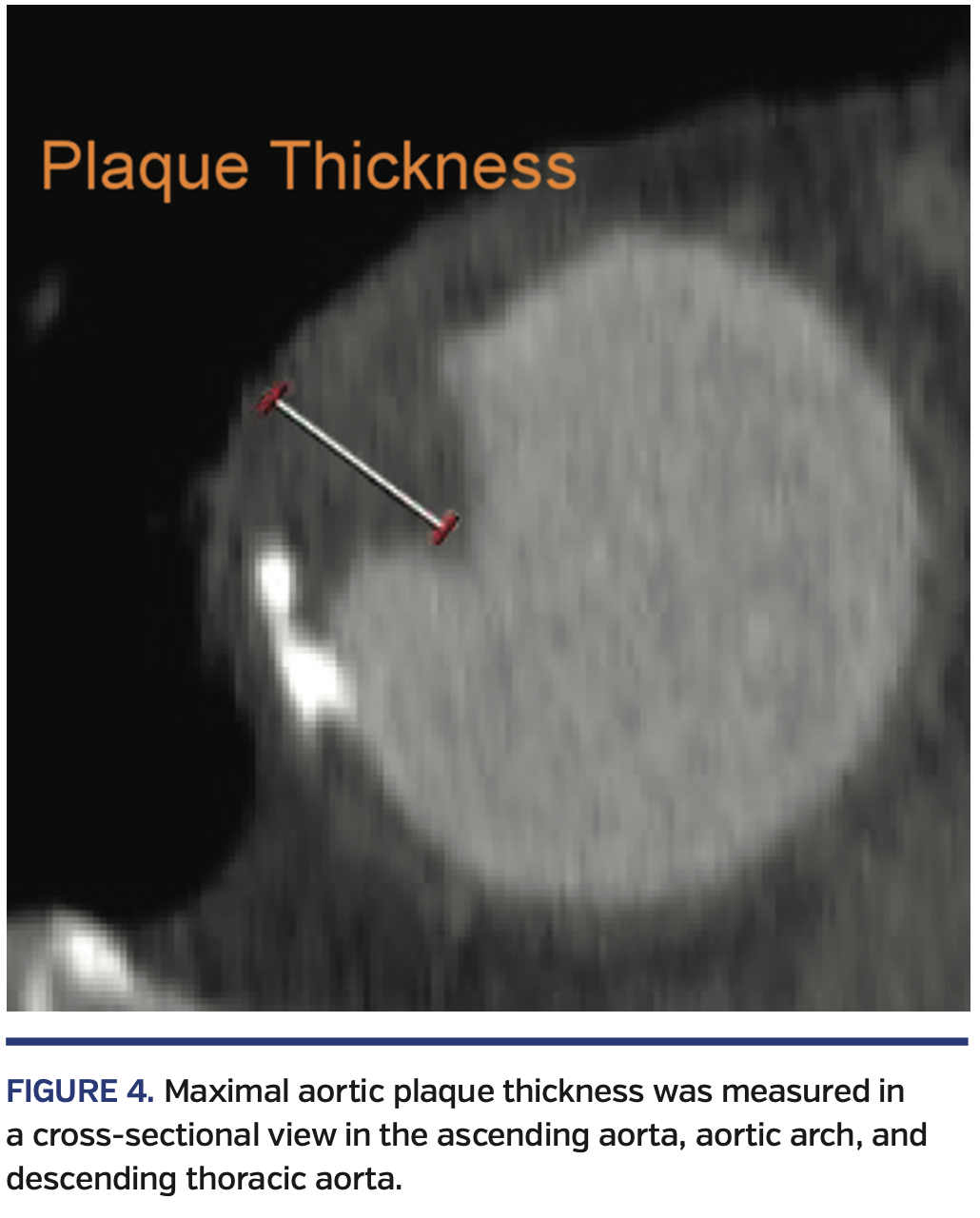
To identify the aortic wall plaque, a line was generated through the centerpoint of the ascending aorta, aortic arch, and descending thoracic aorta. The entire thoracic aorta was then divided into three segments: the ascending aorta between the sinotubular junction (STJ) and the origin of the brachiocephalic artery; the aortic arch between the brachiocephalic artery and the left subclavian artery; and the descending thoracic aorta between the left subclavian artery and the diaphragm. Maximal atheromatous plaque thickness was measured in each segment, perpendicular to the aortic wall (Figure 4).29,36,37
Statistical analyses. Continuous variables were tested for normality of distribution by using the Shapiro-Wilk test and expressed as mean ± standard deviation, or as median and interquartile range (IQR), as appropriate. Either categorical variables or continuous variables were compared using conditional logistic regression analysis. To increase the statistical efficiency given the total number of subjects included in the analysis, propensity-score matching was applied.38 Propensity score was estimated by a logistic regression model using the following factors as the predictors: age (continuous), sex (male/female), body mass index, estimated glomerular filtration rate, year of procedure date, logistic EuroScore (continuous), and the presence or absence of medical history of strokes, transient ischemic attacks, hypertension, diabetes mellitus, dyslipidemia, and peripheral artery disease. Based on the estimated propensity score, we conducted 1:4 matching for patients with and without stroke using 8-to-1 digit matching.39 All analyses were considered significant at a two-tailed P-value of <.05. Propensity-score matching was performed using SAS, version 9.4 (SAS Institute). Other analyses were performed using JMP Pro software, version 12.1.0 (SAS Institute) and SPSS software, version 24.0 (SPSS).
Results
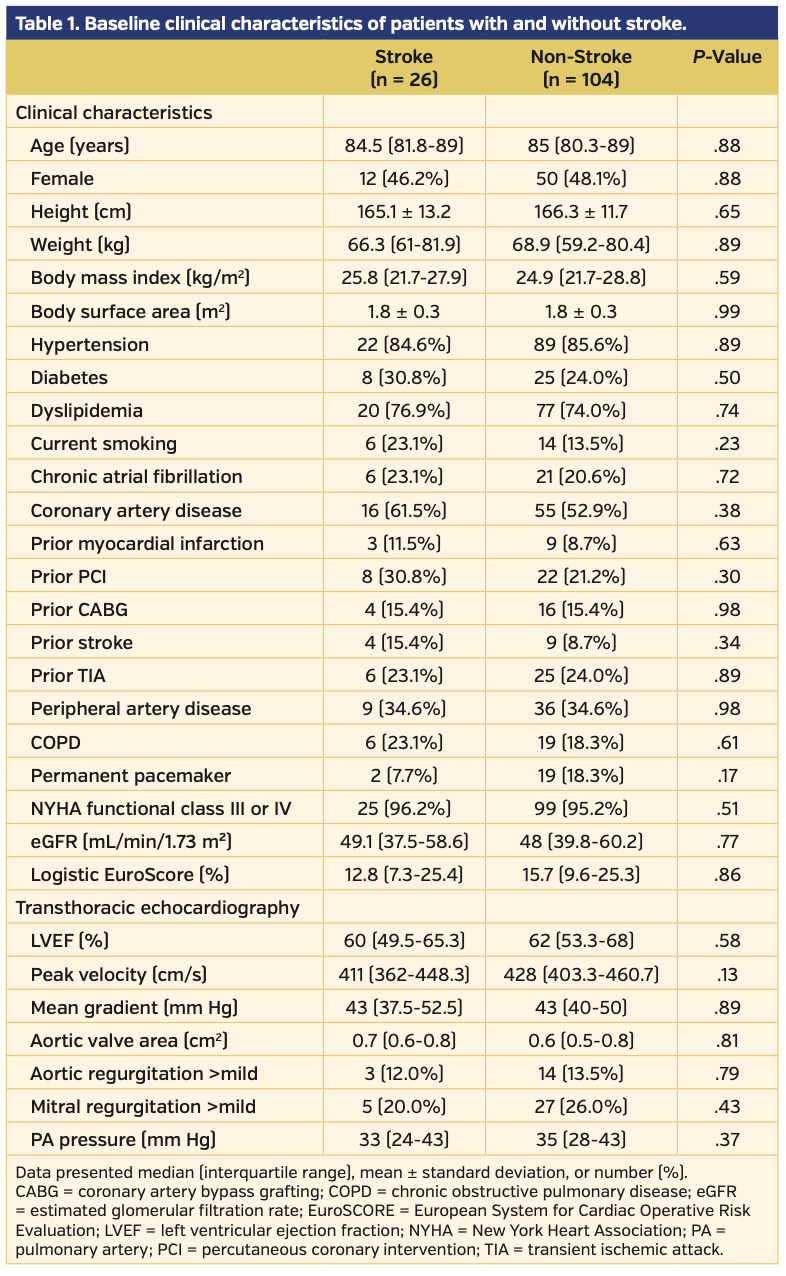
The baseline clinical and echocardiographic characteristics of the entire cohort are summarized in Table 1. The median age was 85 years, and 48% of the patients were female. Prevalence of chronic atrial fibrillation was similar between the two groups (23.1% vs 20.6%; P=.72). There was no difference in the logistic EuroScore or history of hypertension, diabetes, dyslipidemia, stroke, peripheral artery disease, or transient ischemic attack between the two groups. No difference was observed in the left ventricular ejection fraction by transthoracic echocardiography between the two groups.
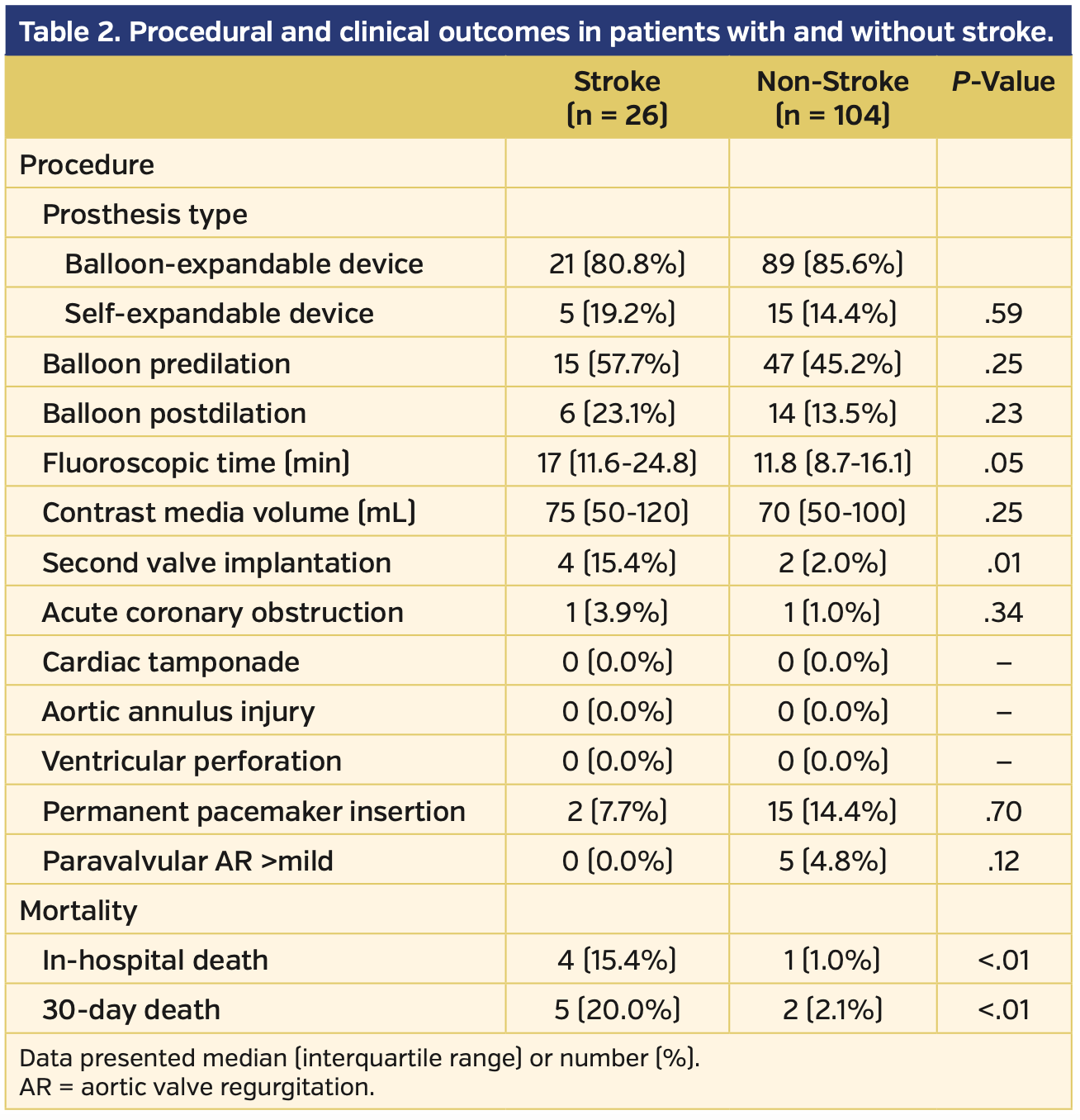
Procedural and clinical outcomes. The procedural and clinical outcomes are listed in Table 2. Patients with periprocedural stroke had a higher incidence of second valve implantation (15.4% vs 2.0%; P=.01), and, accordingly, longer fluoroscopic time (17 min [IQR, 11.6-24.8 min] vs 11.8 min [IQR, 8.7-16.1 min]; P=.05). No significant differences were observed in device type, use of balloon predilation or postdilation, contrast media volume, need for a permanent pacemaker, greater than mild paravalvular aortic regurgitation, or coronary obstruction. There were no cases of aortic annulus injury, ventricular perforation, or cardiac tamponade. In-hospital and 30-day mortality rates were significantly higher in the stroke group (in-hospital mortality, 15.4% vs 1.0% [P<.01] and 30-day mortality, 20.0% vs 2.1% [P<.01]).
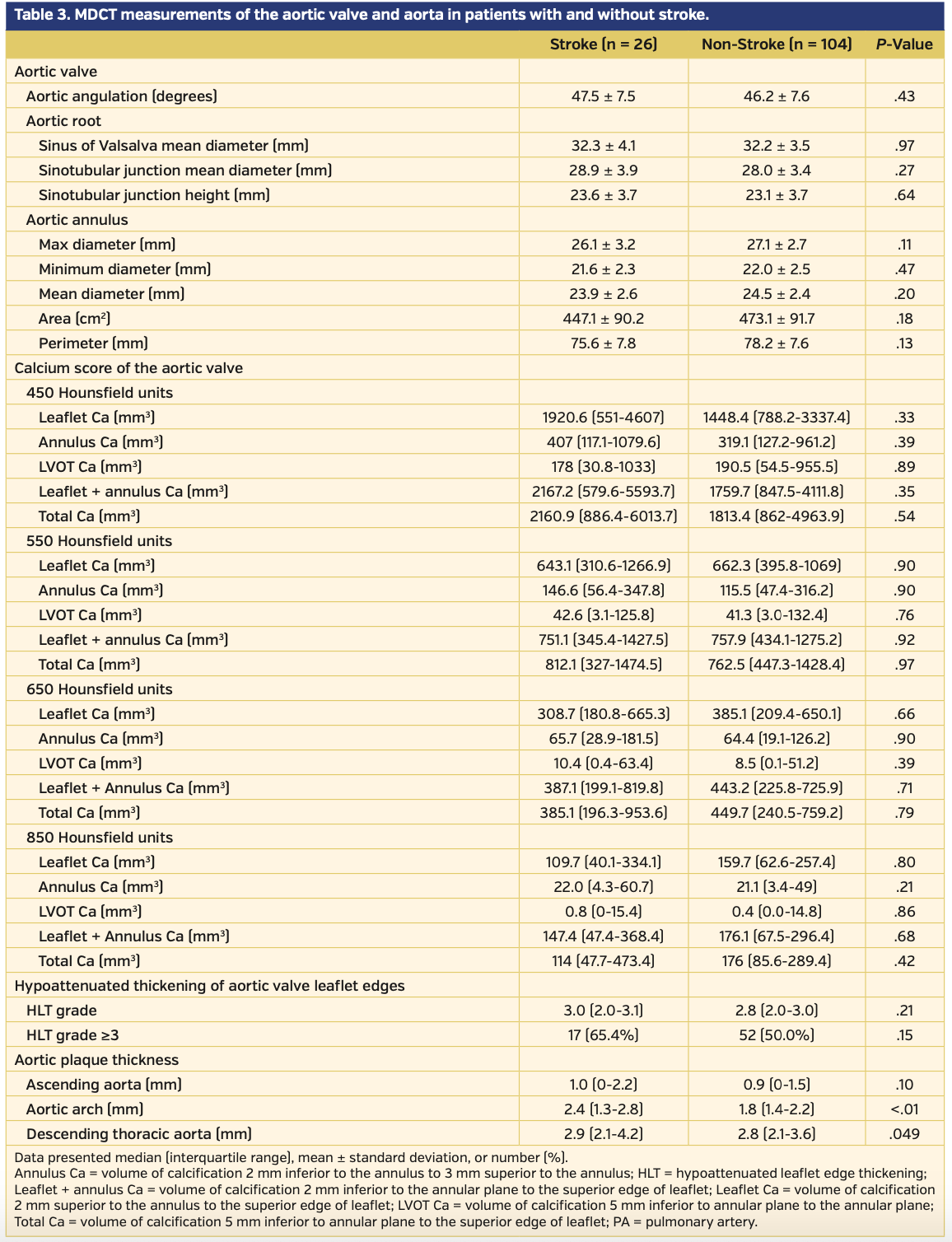
MDCT data. MDCT measurements are listed in Table 3. There were no differences between the two groups in terms of maximum, minimum, and mean aortic diameter, aortic valve area, aortic valve perimeter, mean diameter of the sinus of Valsalva, STJ mean diameter or STJ height, and aortic root angulation. There were no observed differences between the groups in leaflet Ca, annulus Ca, LVOT Ca, leaflet-annulus Ca, or total Ca, regardless of the threshold used (Table 3). The HLT grade was similar between the two groups (3.0 [IQR, 2.0-3.1] vs 2.8 [IQR, 2.0-3.0]; P=.21). The incidence of HLT grade ≥3 was greater in the stroke group than in the non-stroke group (17 [65.4%] vs 52 [50.0%], respectively; P=.15); however, this difference was not statistically significant. The median value of the maximal plaque thicknesses of the aortic wall of the ascending aorta, aortic arch, and descending aorta in the entire cohort was 0.9 mm [IQR, 0-1.5 mm], 1.8 mm [IQR, 1.4-2.4 mm], and 2.8 mm [IQR, 2.1-3.6 mm], respectively. Although the maximal plaque thicknesses of the aortic wall of the ascending aorta was similar between the two groups (ascending aorta: 1.0 mm [IQR, 0-2.2 mm] vs 0.9 mm [IQR, 0-1.5 mm]; P=.10), maximal plaque thicknesses of the aortic wall of the aortic arch, and descending aorta were greater in the stroke group than in the non-stroke group (aortic arch: 2.4 mm [IQR, 1.3-2.8 mm] vs 1.8 mm [IQR, 1.4-2.2 mm]; P<.01; descending aorta: 2.9 mm [IQR, 2.1-4.2 mm] vs 2.8 mm [IQR, 2.1- 3.6 mm]; P=.049).
Discussion
There are two main findings of this study. First, a greater aortic wall plaque thickness detected by preprocedural contrast CT, particularly in the aortic arch, was associated with stroke occurring within 48 hours after TAVR. Second, contrary to our expectation, neither HLT grade nor calcium volume of the aortic valve complex was a predictor of periprocedural stroke.
The results showed that greater aortic plaque thickness of the aorta, detected by preprocedural contrast CT, was associated with stroke occurring within 48 hours after TAVR. In particular, maximal plaque thickness of the aortic arch was strongly associated with stroke (aortic arch: 2.4 mm [IQR, 1.3-2.8 mm] vs 1.8 mm [IQR, 1.4-2.2 mm]; P<.01). However, previous studies have shown conflicting results regarding the association between atheroma of the aortic wall and stroke. Furthermore, the methods for assessing aortic atheroma were different in these studies owing to a lack of consensus regarding the assessment and classification of aortic atheroma.24,29,31 For instance, aortic atheroma assessed by echocardiography was an independent risk factor for asymptomatic strokes detected by MRI24 but was not associated with microembolization detected by transcranial Doppler examination.29 Aortic atheroma volume assessed by CT in the ascending aorta and aortic arch was associated with symptomatic periprocedural stroke;31 however, the method was different from that reported in a previous study that investigated the association between aortic atheroma and ischemic stroke.37 These discrepancies make it difficult to compare the results of this study with previous studies. Nevertheless, the authors believe that the results of this study are valuable because aortic plaque thickness is easily measured by preprocedural contrast CT and can be routinely measured in clinical practice. Further studies to confirm that aortic plaque thickness is an independent predictor of stroke related to TAVR are warranted. This study also revealed that although second valve implantation is not an imaging-based variable but a procedural-related variable, it was observed more frequently in the stroke group. Second valve implantation has not been reported as a predictor of periprocedural stroke. However, repositioning of the THV (or longer fluoroscopic time) was reportedly identified as a risk factor of periprocedural stroke.20 Thus, it can be readily assumed that a complicated manipulation requiring a second valve in a TAVR procedure has a higher chance of causing stroke.
Contrary to our expectation, HLT grade was not a predictive factor of periprocedural stroke. We presumed that hypoattenuated areas of the aortic valve leaflets were indicative of atheroma, fibrosis, or thrombus, although the differentiation was difficult owing to the presence of contrast media, which interfered with the precise HU measurements of the different tissues. Based on previous histopathology studies, stenotic aortic valve leaflets consist of atheroma formed by lipids, fibrotic tissue, and calcium.40,41 Embolic protection studies have shown that the embolic debris captured in a filter-based device during TAVR contains calcification, arterial wall, valve tissue, thrombus, myocardium, and foreign material collagenous tissue.42,43 Thus, it can be postulated that aortic valve tissue is a source of embolization to the brain. In this study, the incidence of HLT grade ≥3 in the stroke group was numerically greater than observed in the non-stroke group (17 [65.4%] vs 52 [50.0%], respectively; P=.15). Given these previous studies and the higher incidence of an HLT grade ≥3 in the stroke group, we cannot deny the possibility of insufficient statistical power to detect a difference in HLT grade between the two study groups. The result that showed no association between the calcium score of the aortic valve complex and periprocedural stroke is consistent with findings from previously published studies that showed a lack of an association between calcium volumes of the aortic valve and stroke related to TAVR.24,44,45
Given the conflicting results regarding the risk factors of periprocedural stroke in previous reports, it should be taken into account that the timing of stroke related to TAVR was different in each previous report. For example, Tay et al46 defined a stroke occurring within 24 hours after TAVR as “immediate stroke,”while Kapadia et al14 defined a stroke occurring within 7 days as “early stroke.” Furthermore, different risk factors were associated with different timings of strokes. For example, Luis et al47 classified a stroke that occurred within 24 hours as “acute stroke” and a stroke that occurred between 1 and 30 days after TAVR as “subacute stroke.” Then, they reported no association between new-onset atrial fibrillation and “acute stroke” in their study,47 although they demonstrated that new-onset atrial fibrillation was a risk factor of “subacute stroke.” Similarly, in some studies investigating an association between stroke and leaflet thrombosis of THV after TAVR, the timing of strokes and CT scanning detecting leaflet thrombosis is an issue.48-51 For example, although cumulative incidence of stroke was assessed in these studies, incidence of acute stroke just after TAVR was not evaluated in these studies. Furthermore, the timing of CT scanning was quite different across the studies. The median time from TAVR to CT scanning was 58 days in the study showing a possible association between stroke and leaflet thrombosis,48 while the median time was 3 days in another study revealing no association between stroke and leaflet thrombosis.49 Considering these facts, the cohort in our study was homogeneous in terms of the timing of stroke occurrence, and this may contribute to identification of the risk factors of acute stroke related to TAVR procedure. Meanwhile, some caution should be exercised in interpreting the results of this study, because we did not include patients who developed stroke after 48 hours.
The method used for measuring aortic wall plaque in this study can be easily and rapidly performed during a routine work-up before TAVR to assess the risk for periprocedural stroke, without the requirement of any additional invasive imaging or expenses. A stroke risk assessment may contribute to better patient selection or treatment strategy, such as the use of embolic protection.
Study limitations. This study has some limitations. First, this was a single-center, retrospective study. Second, the small sample size of this study may be responsible for the failure to detect a statistically significant difference in the HLT grade between the two groups.
Conclusion
The degree of hypoattenuation or calcification of the aortic valve determined by CT was not predictive of stroke in this study. The thickness of the aortic wall plaque measured by contrast-enhanced CT might be a predictive parameter of periprocedural strokes that occur within 48 hours after TAVR.
From the 1Cedars-Sinai Medical Center, Heart Institute, Los Angeles, California; 2Yokohama City University School of Medicine, Department of Data Science, Yokohama, Japan; 3Sendai Kosei Hospital, Cardiovascular Center, Sendai, Japan; and 4Meisei University, Center for Regional Cooperation Iwaki, Iwaki, Japan.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Sharma reports consultant income from Edwards Lifesciences, Abbott Vascular, Keystone Heart, and Boston Scientific. Dr Makkar reports grant support from Edwards Lifesciences; consultant income from Abbott Vascular, Cordis, and Medtronic; equity in Entourage Medical. The remaining authors report no conflicts of interest regarding the content herein.
Manuscript submitted July 6, 2019, provisional acceptance given July 9, 2019, final version accepted July 22, 2019.
Address for correspondence: Raj R. Makkar, MD, Cedars-Sinai Heart Institute, 127 S. San Vicente Blvd, Suite A3421, Los Angeles, CA 90048. Email: Raj.Makkar@cshs.org
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607.
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198.
- Kapadia SR, Leon MB, Makkar RR, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485-2491.
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477-2484.
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609-1620.
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790-1798.
- Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
- Linke A, Wenaweser P, Gerckens U, et al. Treatment of aortic stenosis with a self-expanding transcatheter valve: the international multi-centre ADVANCE study. Eur Heart J. 2014;35:2672-2684.
- Ludman PF, Moat N, de Belder MA, et al. Transcatheter aortic valve implantation in the United Kingdom: temporal trends, predictors of outcome, and 6-year follow-up: a report from the UK transcatheter aortic valve implantation (TAVI) registry, 2007 to 2012. Circulation. 2015;131:1181-1190.
- Reinöhl J, Kaier K, Reinecke H, et al. Effect of availability of transcatheter aortic-valve replacement on clinical practice. N Engl J Med. 2015;373:2438-2447.
- Gilard M, Eltchaninoff H, Iung B, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012;366:1705-1715.
- Miller DC, Blackstone EH, Mack MJ, et al. Transcatheter (TAVR) versus surgical (AVR) aortic valve replacement: occurrence, hazard, risk factors, and consequences of neurologic events in the PARTNER trial. J Thorac Cardiovasc Surg. 2012;143:832-843, e813.
- Généreux P, Head SJ, Mieghem NM, et al. Clinical outcomes after transcatheter aortic valve replacement using Valve Academic Research Consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol. 2012;59:2317-2326.
- Kapadia S, Agarwal S, Miller DC, et al. Insights into timing, risk factors, and outcomes of stroke and transient ischemic attack after transcatheter aortic valve replacement in the PARTNER trial (placement of aortic transcatheter valves). Circ Cardiovasc Interv. 2016;9:e002981.
- Muralidharan A, Thiagarajan K, Van Ham R, et al. Meta-analysis of perioperative stroke and mortality in transcatheter aortic valve Implantation. Am J Cardiol. 2016;118:1031-1045.
- Fanning JP, Wesley AJ, Walters DL, et al. Neurological injury in intermediate-risk transcatheter aortic valve implantation. J Am Heart Assoc. 2016;5.
- Athappan G, Gajulapalli RD, Sengodan P, et al. Influence of transcatheter aortic valve replacement strategy and valve design on stroke after transcatheter aortic valve replacement: a meta-analysis and systematic review of literature. J Am Coll Cardiol. 2014;63:2101-2110.
- Herrmann HC, Thourani VH, Kodali SK, et al. One-year clinical outcomes with SAPIEN 3 transcatheter aortic valve replacement in high-risk and inoperable patients with severe aortic stenosis: clinical perspective. Circulation. 2016;134:130-140.
- Meredith IT, Dumonteil N, Blackman DJ, et al. Repositionable percutaneous aortic valve implantation with the Lotus valve: 30-day and 1-year outcomes in 250 high-risk surgical patients. EuroIntervention. 2017;13:788-795.
- Kleiman NS, Maini BJ, Reardon MJ, et al. Neurological events following transcatheter aortic valve replacement and their predictors. Circ Cardiovasc Interv. 2016;9:e003551.
- Arnold M, Schulz-Heise S, Achenbach S, et al. Embolic cerebral insults after transapical aortic valve implantation detected by magnetic resonance imaging. JACC Cardiovasc Interv. 2010;3:1126-1132.
- Kahlert P, Knipp SC, Schlamann M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation. 2010;121:870-878.
- Rodés-Cabau J, Kahlert P, Neumann F-J, et al. Feasibility and exploratory efficacy evaluation of the Embrella embolic deflector system for the prevention of cerebral emboli in patients undergoing transcatheter aortic valve replacement: the PROTAVI-C pilot study. JACC Cardiovasc Interv. 2014;7:1146-1155.
- Uddin A, Fairbairn TA, Djoukhader IK, et al. Consequence of cerebral embolism after transcatheter aortic valve implantation compared with contemporary surgical aortic valve replacement: effect on health-related quality of life. Circ Cardiovasc Interv. 2015;8:e001913.
- Ghanem A, Muller A, Nahle CP, et al. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion-weighted magnetic resonance imaging. J Am Coll Cardiol. 2010;55:1427-1432.
- Auffret V, Regueiro A, Trigo M, et al. Predictors of early cerebrovascular events in patients with aortic stenosis undergoing transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:673-684.
- Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, Min JK, Leipsic JA. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr. 2012;6:366-380.
- Bax JJ, Delgado V, Bapat V, et al. Open issues in transcatheter aortic valve implantation. Part 1: patient selection and treatment strategy for transcatheter aortic valve implantation. Eur Heart J. 2014;35:2627-2638.
- Kahlert P, Al-Rashid F, Dottger P, et al. Cerebral embolization during transcatheter aortic valve implantation: a transcranial Doppler study. Circulation. 2012;126:1245-1255.
- Erdoes G, Basciani R, Huber C, et al. Transcranial Doppler-detected cerebral embolic load during transcatheter aortic valve implantation. Eur J Cardiothorac Surg. 2012;41:778-783; discussion 783-774.
- Kataoka Y, Puri R, Pisaniello AD, et al. Aortic atheroma burden predicts acute cerebrovascular events after transcatheter aortic valve implantation: insights from volumetric multislice computed tomography analysis. EuroIntervention. 2016;12:783-789.
- Thambidorai SK, Jaffer SJ, Shah TK, Stewart WJ, Klein AL, Lauer MS. Association of atheroma as assessed by intraoperative transoesophageal echocardiography with long-term mortality in patients undergoing cardiac surgery. Eur Heart J. 2007;28:1454-1461.
- Jilaihawi H, Kashif M, Fontana G, et al. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol. 2012;59:1275-1286.
- Khalique OK, Hahn RT, Gada H, et al. Quantity and location of aortic valve complex calcification predicts severity and location of paravalvular regurgitation and frequency of post-dilation after balloon-expandable transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2014;7:885-894.
- Jilaihawi H, Makkar RR, Kashif M, et al. A revised methodology for aortic-valvar complex calcium quantification for transcatheter aortic valve implantation. Eur Heart J Cardiovasc Imaging. 2014;15:1324-1332.
- Amarenco P, Cohen A, Hommel M, et al; French Study of Aortic Plaques in Stroke Group. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med. 1996;334:1216-1221.
- Lee K, Hur J, Hong SR, et al. Predictors of recurrent stroke in patients with ischemic stroke: comparison study between transesophageal echocardiography and cardiac CT. Radiology. 2015;276:381-389.
- Rosenum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41-55.
- Parsons LS. Performing a 1:N case-control match on propensity score. Presented at the 29th Annual SAS Users Group International Conference. Montreal, Canada; 2004.
- Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol. 1999;19:1218-1222.
- Rajamannan NM, Evans FJ, Aikawa E, et al. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: calcific aortic valve disease-2011 update. Circulation. 2011;124:1783-1791.
- Mieghem NM, Schipper M, Ladich E, et al. Histopathology of embolic debris captured during transcatheter aortic valve replacement: clinical perspective. Circulation. 2013;127:2194-2201.
- Kapadia SR, Kodali S, Makkar R, et al. Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol. 2017;69:367-377.
- Fairbairn TA, Mather AN, Bijsterveld P, et al. Diffusion-weighted MRI determined cerebral embolic infarction following transcatheter aortic valve implantation: assessment of predictive risk factors and the relationship to subsequent health status. Heart. 2012;98:18-23.
- Staubach S, Franke J, Gerckens U, et al. Impact of aortic valve calcification on the outcome of transcatheter aortic valve implantation: results from the prospective multicenter German TAVI registry. Catheter Cardiovasc Interv. 2013;81:348-355.
- Tay ELW, Gurvitch R, Wijesinghe N. A high-risk period for cerebrovascular events exists after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2011;4:1290-1297.
- Nombela-Franco L, Webb JG, de Jaegere PP, et al. Timing, predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation. 2012;126:3041-3053.
- Chakravarty T, Sondergaard L, Friedman J, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. 2017;389:2383-2392.
- Yanagisawa R, Tanaka M, Yashima F, et al. Early and late leaflet thrombosis after transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2019;12:e007349.
- Vollema EM, Kong WKF, Katsanos S, et al. Transcatheter aortic valve thrombosis: the relation between hypo-attenuated leaflet thickening, abnormal valve haemodynamics, and stroke. Eur Heart J. 2017;38:1207-1217.
- Makkar RR, Fontana G, Jilaihawi H, et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med. 2015;373:2015-2024.