Intravascular Ultrasound Assessment of Postprocedural Incomplete Stent Apposition
Abstract: Background. There has been no detailed intravascular ultrasound (IVUS) analysis to evaluate the degree to which stent underexpansion or reference vessel/stent size mismatch contributes to the occurrence of post-procedural incomplete stent apposition (post-ISA). Methods. We evaluated 238 lesions treated with everolimus-eluting stents (n = 110) or paclitaxel-eluting stents (n = 128). Reference lumen/stent area ratio was defined as the ratio of lumen area adjacent to the stent edge in the reference segment to stent area at the stent edge or at stent body ISA site. Results. Post-ISA was observed in 36 of the 238 cases (15%) at the proximal stent edge, 15 of the 238 cases (6%) at the distal stent edge and 14 of the 238 cases (6%) at stent body. Reference lumen/stent area ratio was significantly greater in the ISA group compared with non-ISA in proximal edge (127 ± 20 vs 99 ± 10%; P<.001), and greater reference lumen/stent area ratio (118 ± 18 vs 94 ± 11%; P<.001) and higher presence of calcification (60 vs 29%; P<0.001) were observed in distal edge ISA group compared with non-ISA. At the stent body, presence of calcification was more frequently observed in the ISA compared with the non-ISA group (86 vs 42%; P=.002). Conclusions. Post-ISA at the stent edge was significantly associated with vessel/stent mismatch rather than stent underexpansion. IVUS-guided appropriate stent or balloon sizing might be useful to prevent post-ISA and optimize initial stent deployment.
J INVASIVE CARDIOL 2012;24(1):13-16
Key words: apposition, balloon sizing, ISA, intravascular ultrasound
_________________________________________
Incomplete stent apposition (ISA) is defined as a lack of contact between stent struts and the underlying vessel wall.1 Post-procedural ISA (post-ISA), which is observed immediately after stent deployment, may resolve as the stent struts become covered due to neointimal hyperplasia during the follow-up period. However, in some cases, follow-up intravascular ultrasound (IVUS) examination shows persistent-ISA, defined by the existence of ISA in the same location of post-ISA even several months after stent deployment. Although the clinical impact of post- and persistent-ISA are still in dispute,2-7 a retrospective IVUS registry previously reported that ISA was identified with subsequent stent thrombosis.8
The mechanisms of post-ISA are associated with multiple factors including clinical (male gender, myocardial infarction), lesion-related (calcification), stent-related (material, design) and technique-dependent.9-13 Of the above, the technique-related factors, consisting of stent underexpansion and reference vessel/stent size mismatch, have not been scrutinized by targeted and detailed IVUS analysis. Understanding the exact mechanism of post-ISA may translate to improved clinical benefit via decreased rate of post-ISA. Therefore, the aims of this study were to evaluate the mechanisms of post-ISA using IVUS.
Methods
Patient population. All data were derived from the Corelab database using previous multicenter, randomized trials: ZoMaxx I, ZoMaxx II, and SPIRIT III.14-18 According to the research protocol for each clinical trial, IVUS interrogation was planned for all the patients at prespecified enrollment sites at postprocedure and at 8-9 months after stent implantation. Patients who met the following criteria were enrolled in this study: (1) implantation of either everolimus-eluting stent (EES) or paclitaxel-eluting stent (PES); (2) native coronary artery lesion; and (3) availability of high-quality IVUS images with volumetric analysis at baseline (postintervention). Lesions with a branch adjacent to the stent edge in the reference segment and multistent lesions using different stent sizes with adjunctive balloon postdilatation were excluded. Stent implantation was performed using current conventional techniques using IVUS; however, the intervention strategy, including multiple postdilatations, was left to the discretion of the operator.
IVUS analysis. All IVUS images were acquired and analyzed using EchoPlaque dedicated software (INDEC Systems, Inc.). Post-ISA was defined as >1 stent strut clearly separated from the vessel wall with evidence of blood speckle behind the strut without overlapping a sidebranch. Post-ISA was classified into 3 groups according to ISA location: proximal edge ISA (within 3 mm from proximal stent edge), distal edge ISA (within 3 mm from distal stent edge), and stent body ISA.
Stent area at the stent edge and lumen area adjacent to the stent edge in the reference segment (within 1 mm from the stent edge) were measured using a single still image. Stent expansion was defined as the ratio of stent area at the stent edge or at stent body ISA site to predicted stent area calculated from final balloon diameter. Reference lumen/stent area ratio was defined as the ratio of lumen area adjacent to the stent edge in the reference segment to stent area at the stent edge or at stent body ISA site. To obtain stent expansion and reference lumen/stent area ratio in non-ISA group, single cross-sectional images at the stent edge or at the center of stent body were used.
Statistics. Categorical variables were presented as counts and percentages. Continuous variables were presented as mean values ± standard deviation (SD). Categorical variables were compared with Fisher exact test or chi-square test. For continuous variables, unpaired student’s t-test was used to differentiate between two sets of data. Baseline angiographic, procedural characteristics, and IVUS findings were incorporated into a logistic regression model to assess the independent contribution of parameters to post-ISA. The partial correlation coefficient test was used to assess which variables should be included in the model, with coefficient <0.6 required for entry. Baseline factors used for the analysis were location of coronary lesion, type of stents, number of stents per lesion, stent diameter, adjunctive balloon post-dilatation, maximal inflation pressure, stent expansion, reference lumen/stent area ratio, and existence of calcification. P<.05 indicated statistical significance.
Results
 Baseline characteristics of lesions with and without post-ISA. Among a total of 238 cases reviewed (EES, n = 110; PES, n = 128), 54 cases (23%) showed at least one post-ISA. Baseline patient characteristics were not significantly different between the ISA and non-ISA groups. Longer stent length, and larger maximum balloon diameter and reference diameter were observed in the ISA group; however, other angiographic and procedural characteristics were not different between the 2 groups (Table 1).
Baseline characteristics of lesions with and without post-ISA. Among a total of 238 cases reviewed (EES, n = 110; PES, n = 128), 54 cases (23%) showed at least one post-ISA. Baseline patient characteristics were not significantly different between the ISA and non-ISA groups. Longer stent length, and larger maximum balloon diameter and reference diameter were observed in the ISA group; however, other angiographic and procedural characteristics were not different between the 2 groups (Table 1).
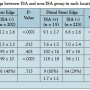 Comparison of IVUS parameters between ISA and non-ISA. Post-ISA was observed in 36 of the 238 cases (15%) at the proximal stent edge and in 15 of the 238 cases (6%) at the distal stent edge. Multiple post-ISAs at both proximal and distal stent edge were observed in 5 cases (2%). In terms of stent edge ISA, stent expansion ratio was similar or even higher in stents with ISA than without ISA. Reference lumen/stent area ratio was significantly greater in the ISA group for both proximal and distal ISA cases compared with the non-ISA group. While the incidence of calcification in the proximal reference segment did not differ between the ISA and non-ISA cases, distal edge ISA showed significantly higher in the ISA group compared with the non-ISA group (Table 2). Multiple logistic regression analysis showed reference lumen/stent area ratio was identified to be the independent risk factor for proximal edge ISA (odds ratio [OR], 1.24; 95% confidential interval [CI], 1.15-1.34; P<.0001). With respect to distal edge ISA, reference lumen/stent area ratio and the existence of calcification were identified as the independent risk factors (OR, 1.36 and 15.5; 95% CI, 1.14-1.60 and 1.84-130.22; P<.001 and P=.012, respectively).
Comparison of IVUS parameters between ISA and non-ISA. Post-ISA was observed in 36 of the 238 cases (15%) at the proximal stent edge and in 15 of the 238 cases (6%) at the distal stent edge. Multiple post-ISAs at both proximal and distal stent edge were observed in 5 cases (2%). In terms of stent edge ISA, stent expansion ratio was similar or even higher in stents with ISA than without ISA. Reference lumen/stent area ratio was significantly greater in the ISA group for both proximal and distal ISA cases compared with the non-ISA group. While the incidence of calcification in the proximal reference segment did not differ between the ISA and non-ISA cases, distal edge ISA showed significantly higher in the ISA group compared with the non-ISA group (Table 2). Multiple logistic regression analysis showed reference lumen/stent area ratio was identified to be the independent risk factor for proximal edge ISA (odds ratio [OR], 1.24; 95% confidential interval [CI], 1.15-1.34; P<.0001). With respect to distal edge ISA, reference lumen/stent area ratio and the existence of calcification were identified as the independent risk factors (OR, 1.36 and 15.5; 95% CI, 1.14-1.60 and 1.84-130.22; P<.001 and P=.012, respectively).
Post-ISA at the stent body was observed in 14 lesions (6%). There were no significant differences in terms of stent expansion and reference lumen/stent area ratio between the ISA and non-ISA group. However, the incidence of calcification was significantly higher in the ISA compared with non-ISA group (Table 2).
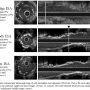 Comparison of IVUS parameters among different ISA locations. Reference lumen/stent area ratio in the proximal ISA group was significantly greater than that in the stent body ISA group (P=.048). The incidence of calcification was significantly different among varying locations of post-ISA and highest in the body ISA group (P=.029). Maximum ISA area was larger in the proximal edge ISA compared with the stent body ISA group (P=.034). However, stent area and stent expansion were not significantly different among the 3 groups. Representative images of post-ISA lesions are shown in Figure 1.
Comparison of IVUS parameters among different ISA locations. Reference lumen/stent area ratio in the proximal ISA group was significantly greater than that in the stent body ISA group (P=.048). The incidence of calcification was significantly different among varying locations of post-ISA and highest in the body ISA group (P=.029). Maximum ISA area was larger in the proximal edge ISA compared with the stent body ISA group (P=.034). However, stent area and stent expansion were not significantly different among the 3 groups. Representative images of post-ISA lesions are shown in Figure 1.
Discussion
The main findings of this study were: (1) at the proximal stent edge, reference lumen/stent area ratio was significantly associated with post-ISA; and (2) at the distal stent edge, both reference lumen/stent area ratio and the presence of calcification were significantly associated with post-ISA.
Stent cell design and strut thickness were reported to correlate with apposition of drug-eluting stents assessed by optical coherence tomography, which has 10-times higher imaging resolution than IVUS.10 In the present IVUS study, stent type was not significantly associated with post-ISA either at the proximal or distal stent edge. IVUS could not detect such a small difference of stent apposition among stents. In addition, even for the same type of stent, the incidence of post-ISA assessed by IVUS varied widely among previously reported trials: 16.2-19.1%1,19 in the Cypher sirolimus-eluting stent (Cordis Corporation) and 12.6-24.2% in the Endeavor zotarolimus-eluting stent (Medtronic).19-21 The inconsistent results likely suggest a significant contribution of other factors to the occurrence of ISA, such as the technique-related factors, rather than stent cell design and strut thickness.13
As there had been no systematic analysis to elucidate mechanisms of post-ISA at the stent edge, it was unclear whether stent underexpansion or reference vessel/stent size mismatch contributed to the occurrence of post-ISA at the stent edge. In this study, the stent expansion ratio was similar or even higher in the ISA group compared to the non-ISA group, suggesting that stents were well-expanded in the ISA group. On the other hand, the reference lumen/stent area ratio was significantly greater in the ISA group than in the non-ISA group, suggesting that greater reference vessel/stent size mismatch was observed in the ISA group. Multivariate logistic regression analysis revealed that reference lumen/stent area ratio was significantly associated with the incidence of post-ISA at both stent edges. In view of these results, post-ISA at the stent edge might be due to reference vessel/stent size mismatch rather than stent underexpansion. In other words, stent struts were not well apposed to the vessel wall, even though the stent was well expanded to the nominal balloon size. Therefore, IVUS-guided appropriate stent sizing or post-stent deployment balloon dilatation using an appropriate balloon size may decrease the rate of post-ISA at the stent edge.
Reference/stent size mismatch was significantly associated with post-ISA at the distal stent edge. The existence of calcification was also associated with it at the distal stent edge. These findings were consistent with a previous study showing that extensive calcification of coronary arteries was associated with more frequent post-ISA.12 One might expect that a stent underexpansion due to severe calcification contributes to the occurrence of ISA at the distal stent edge. However, in this study, the partial correlation coefficient between the stent underexpansion and calcification was not significant (data not shown) and ISA at the distal stent edge was associated with existence of calcification, but not stent underexpansion. The exact relationship between ISA at the distal stent edge and calcification needs further investigation.
Post-ISA may potentially prevent or delay endothelialization of the stent struts due to lack of contact with the vessel wall.22 A retrospective IVUS registry suggested that post-ISA is associated with subsequent stent thrombosis.8 Conversely, other investigators have reported that post-ISA was not related to adverse clinical events.2-7 However, considering the low incidence of stent thrombosis, it is possible that the potential impact of ISA may have been underestimated in stent thrombosis.23-25 Understanding the mechanisms of post-ISA may be important in the clinical setting because a reduction in post-ISA may confer better clinical outcomes through a possible reduction of stent thrombosis.
Limitations. Several limitations should be noted. First, this is a retrospective study based on a relatively limited sample size, raising the possibility of selection bias. Second, we evaluated only postprocedural IVUS images following stent implantation. Therefore, the relationship between plaque distribution, composition data before stent deployment and post-ISA was not evaluated. Third, the mechanisms of post-ISA might be associated with multiple factors including clinical, lesion-related, stent-related, and technique-dependent. Further detailed analysis with a large population will be needed to assess the exact mechanisms of postprocedural ISA.
Conclusion
Post-ISA at the stent edge was significantly associated with vessel/stent mismatch rather than stent underexpansion. IVUS-guided appropriate stent or balloon sizing might be useful to prevent post-ISA and optimize initial stent deployment.
References
- Ako J, Morino Y, Honda Y, et al. Late incomplete stent apposition after sirolimus-eluting stent implantation: a serial intravascular ultrasound analysis. J Am Coll Cardiol. 2005;46(6):1002-1005.
- Steinberg DH, Mintz GS, Mandinov L, et al. Long-term impact of routinely detected early and late incomplete stent apposition: an integrated intravascular ultrasound analysis of the TAXUS IV, V, and VI and TAXUS ATLAS workhorse, long lesion, and direct stent studies. JACC Cardiovasc Interv. 2010;3(5):486-494.
- Kimura M, Mintz GS, Carlier S, et al. Outcome after acute incomplete sirolimus-eluting stent apposition as assessed by serial intravascular ultrasound. Am J Cardiol. 2006;98(4):436-442.
- Fujii K, Carlier SG, Mintz GS, et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol. 2005;45(7):995-998.
- Degertekin M, Serruys PW, Tanabe K, et al. Long-term follow-up of incomplete stent apposition in patients who received sirolimus-eluting stent for de novo coronary lesions: An intravascular ultrasound analysis. Circulation. 2003;108(22):2747-2750.
- Tanabe K, Serruys PW, Degertekin M, et al. Incomplete stent apposition after implantation of paclitaxel-eluting stents or bare metal stents: Insights from the randomized TAXUS II trial. Circulation. 2005;111(7):900-905.
- Hong MK, Mintz GS, Lee CW, et al. Incidence, mechanism, predictors, and long-term prognosis of late stent malapposition after bare-metal stent implantation. Circulation. 2004;109(7):881-886.
- Uren NG, Schwarzacher SP, Metz JA, et al. Predictors and outcomes of stent thrombosis: an intravascular ultrasound registry. Eur Heart J. 2002;23(2):124-132.
- Gonzalo N, Barlis P, Serruys PW, et al. Incomplete stent apposition and delayed tissue coverage are more frequent in drug-eluting stents implanted during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction than in drug-eluting stents implanted for stable/unstable angina: insights from optical coherence tomography. JACC Cardiovasc Interv. 2009;2(5):445-452.
- Tanigawa J, Barlis P, Dimopoulos K, Dalby M, Moore P, Di Mario C. The influence of strut thickness and cell design on immediate apposition of drug-eluting stents assessed by optical coherence tomography. Int J Cardiol. 2009;134(2):180-188.
- Kim YS, Koo BK, Seo JB, et al. The incidence and predictors of postprocedural incomplete stent apposition after angiographically successful drug-eluting stent implantation. Catheter Cardiovasc Interv. 2009;74(1):58-63.
- Mosseri M, Satler LF, Pichard AD, Waksman R. Impact of vessel calcification on outcomes after coronary stenting. Cardiovasc Revasc Med. 2005;6(4):147-153.
- Gerber R, Colombo A. Does IVUS guidance of coronary interventions affect outcome? A prime example of the failure of randomized clinical trials. Catheter Cardiovasc Interv. 2008;71(5):646-654.
- Chevalier B, Di Mario C, Neumann FJ, et al. A randomized, controlled, multicenter trial to evaluate the safety and efficacy of zotarolimus- versus paclitaxel-eluting stents in de novo occlusive lesions in coronary arteries. The ZoMaxx I trial. JACC Cardiovasc Interv. 2008;1(5):524-532.
- Gray WA, Yeung AC, Cutlip DE, et al. A randomized, controlled, multi-center trial comparing the safety and efficacy of zotarolimus-eluting and paclitaxel-eluting stents in de novo lesions in coronary arteries: final results of the ZoMaxx II trial. Int J Cardiol. 2011 Jun 7. (Epub ahead of print).
- Waseda K, Hasegawa T, Ako J, et al. Comparison of vascular response to zotarolimus-eluting stent vs paclitaxel-eluting stent implantation: pooled IVUS results from the ZoMaxx I and II trials. Circ J. 2010;74(11):2334-2339.
- Stone GW, Midei M, Newman W, et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008;299(16):1903-1913.
- Shimohama T, Ako J, Yamasaki M, et al. SPIRIT III JAPAN versus SPIRIT III USA: A comparative intravascular ultrasound analysis of the everolimus-eluting stent. Am J Cardiol. 2010;106(1):13-17.
- Miyazawa A, Ako J, Hongo Y, et al. Comparison of vascular response to zotarolimus-eluting stent versus sirolimus-eluting stent: intravascular ultrasound results from ENDEAVOR III. Am Heart J. 2008;155(1):108-113.
- Sakurai R, Hongo Y, Yamasaki M, et al. Detailed intravascular ultrasound analysis of Zotarolimus-eluting phosphorylcholine-coated cobalt-chromium alloy stent in de novo coronary lesions (results from the ENDEAVOR II trial). Am J Cardiol. 2007;100(5):818-823.
- Waseda K, Miyazawa A, Ako J, et al. Intravascular ultrasound results from the ENDEAVOR IV trial: Randomized comparison between zotarolimus- and paclitaxel-eluting stents in patients with coronary artery disease. JACC Cardiovasc Interv. 2009;2(8):779-784.
- Ozaki Y, Okumura M, Ismail TF, et al. The fate of incomplete stent apposition with drug-eluting stents: an optical coherence tomography-based natural history study. Eur Heart J. 2010;31(12):1470-1476.
- Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315-1323.
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126-2130.
- Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350(3):221-231.
_________________________________________
From the Center for Cardiovascular Technology, Division of Cardiovascular Medicine, Stanford University Medical Center, Stanford, California.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Fitzgerald has received honoraria from Boston Scientific and research grants from Abbott Corporation. No other authors have reported conflicts of interest regarding the content herein.
Manuscript submitted June 27, 2011, provisional acceptance given July 25, 2011, final version accepted October 11, 2011.
Address for correspondence: Yasuhiro Honda, MD, Cardiovascular Medicine, Stanford University, 300 Pasteur Dr, Room H3554, Stanford, CA 94305-5637. Email: crci-cvmed@stanford.edu












