Interaction Between Self-Expanding Transcatheter Heart Valves and Coronary Ostia: An Angiographically Based Analysis of the Evolut R/Pro Valve System
Abstract: Objectives. We sought to assess the position of the CoreValve Evolut R/Pro (Medtronic) with respect to the left coronary artery (LCA) ostium and evaluate the impact of implantation depth on this relationship. Methods. One hundred consecutive patients who received an Evolut R/Pro valve and had an adequate angiography following valve implantation were included. Angiographic measurements included valve implantation depth, the position of the Evolut R/Pro with respect to the LCA, and the distance between the neo-valve cusp and the LCA ostium. Coronary access issues following TAVR were also recorded. Results. Regarding the LCA ostium, the neo-valve of the Evolut R/Pro was supraostial, at the ostial level, and infraostial in 3%, 12%, and 85% of cases, respectively. When beneath the LCA ostium, the mean distance between the neo-valve and the floor of the LCA ostium was 4.1 ± 5.2 mm. An implantation depth ≤6 mm was associated with a higher rate of neo-valve at the ostial level or above (25% vs 4% for implantation depth >6 mm; P=.01). Accessing the coronary arteries was required in 10% of the patients at 12 ± 8 months post TAVR, and selective coronary angiography of the left and right coronary arteries was achieved in 60% and 40% of the cases, respectively. Conclusions. The Evolut R/Pro neo-valve was positioned below the LCA ostium in the vast majority of cases (85%), but an implantation depth ≤6mm was associated with a higher rate of neo-valve positioning at or above the coronary ostia level. Considering the current tendency of very high (aortic) valve implants to avoid conduction disturbances, future studies should determine the impact of high Evolut R/Pro positioning on coronary access issues post TAVR.
Key words: coronary angiogram, percutaneous coronary intervention, transcatheter aortic valve replacement
The recent publication of both the PARTNER 3 trial1 and the Evolut Low Risk trial2 has determined a shift toward the endovascular treatment of low-risk patients who suffer from severe symptomatic aortic stenosis. In this younger and healthier population, the occurrence of conduction disturbances and coronary access following transcatheter aortic valve replacement (TAVR) have become two of the most important topics in the field.
Many reports showed that the use of some self-expanding transcatheter heart valve (THV) systems led to a higher rate of conduction disturbances compared with the balloon-expandable design.3 Therefore, some teams have made efforts to limit valve implantation depth in order to limit bundle of His injury.4 A very high (aortic) THV positioning would likely translate into a reduction of the conduction disturbance rate, but it may increase the risk of valve tissue hindering access to the coronary ostia. It is well known that the stent frame of the CoreValve/Evolut valve system (Medtronic) invariably covers the coronary ostia as it has a supra-annular design,5 but few data exist on the interaction between the neo-valve tissue and the coronary ostia. Thus, the purposes of this analysis were: (1) to assess the position of the neo-valve of the Evolut R/Pro system with respect to left coronary artery (LCA) ostium; and (2) to determine the impact of valve implantation depth on neo-valve/coronary ostia interaction.
Methods
The main characteristics of the Evolut R and Evolut Pro valves according to size are presented in Table 1 and Figure 1.
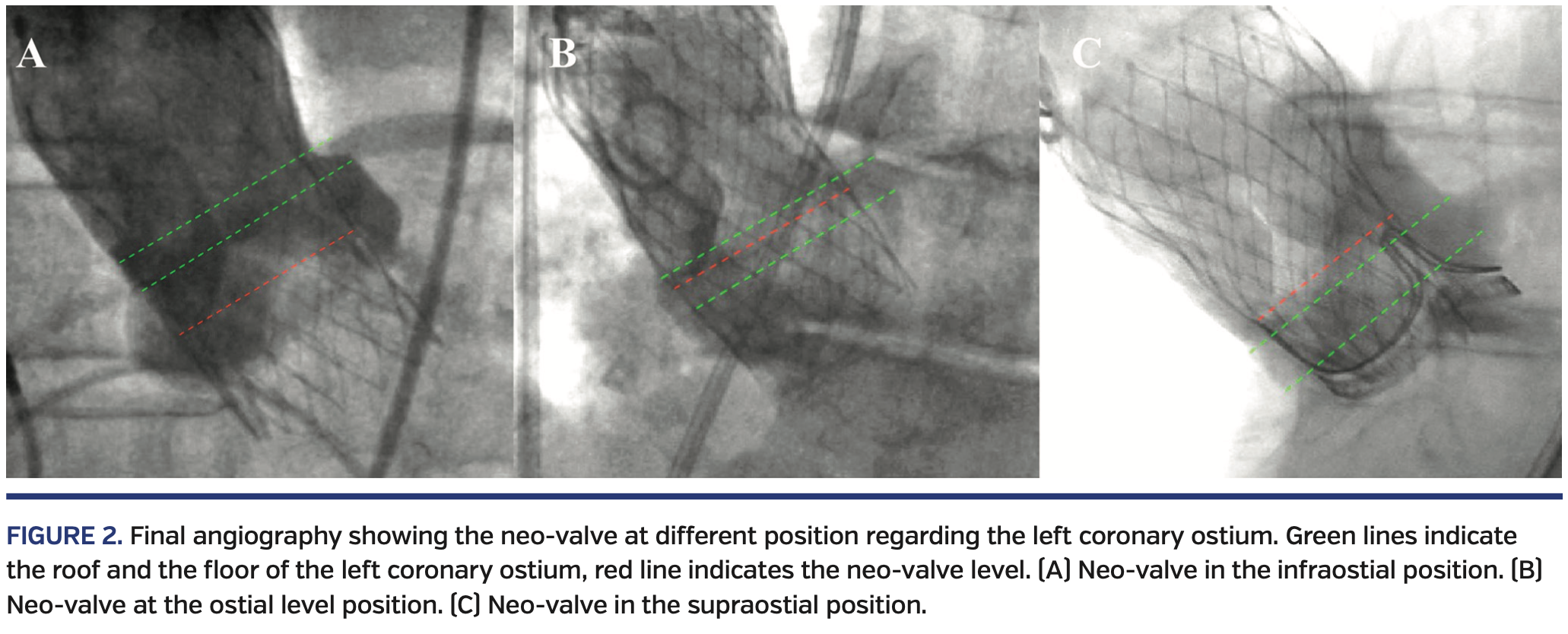
Angiographic analysis. A total of 203 consecutive patients who had severe symptomatic aortic stenosis and were treated with either the Evolut R THV (n = 176) or Evolut Pro THV (n = 27) at our center from February 2015 to September 2019 were screened for eligibility. All angiographies following Evolut R/Pro valve implantation (at the end of the TAVR procedure) were reviewed by two experienced interventional cardiologists. Circumstances such as a non-orthogonal view that precluded an adequate evaluation of THV position in regard to the LCA ostium (n = 36) or patients without final angiographic control (n = 62) were excluded to ensure optimal assessment of valve position. One patient with valve embolization and valve misplacement that required a second THV was also excluded. Finally, a total of 100 patients with adequate angiographic images post valve implantation were included in the analysis and constituted the study population. The position of the THV with respect to the LCA was assessed, and the distance between the neo-valve cusp and the roof or the floor of the LCA ostium was also measured when appropriate. Valve position in regard to the coronary ostia was classified as either supraostial, at the ostial level, or infraostial, with the neo-valve cusp as the reference point (Figure 2). The total expanded valve height was also measured and implantation depth was presented in relative and absolute terms (Figure 3). The orthogonal view of the aortography rarely allowed an acceptable view of the right coronary artery (RCA) ostium, and RCA opacification was frequently inadequate to assess the THV position regarding the RCA ostium; these two factors explain why the position of the RCA in relation to the THV was not included in our analysis. All angiographic measurements were entered into a dedicated database for analyses.
Coronary angiogram and percutaneous coronary intervention post TAVR. Patients who underwent coronary angiogram and/or percutaneous coronary intervention (PCI) after TAVR were identified. Procedural characteristics, including the type and number of catheters used, injection selectivity, performance indicators, and PCI success rate, were recorded.
Statistical analysis. Qualitative variables are expressed as number (percentage), and continuous data as mean (standard deviation) or median (interquartile range [IQR]) according to variable distribution. Categorical variables were compared using the Chi-square or Fisher’s exact test, as appropriate. Statistical analyses were performed with SAS, version 9.4 (SAS Institute).
Results
Patient characteristics. The mean age of the study population was 80 ± 8 years, 62% were women, and the mean STS-PROM score was 5.0 ± 2.8%.
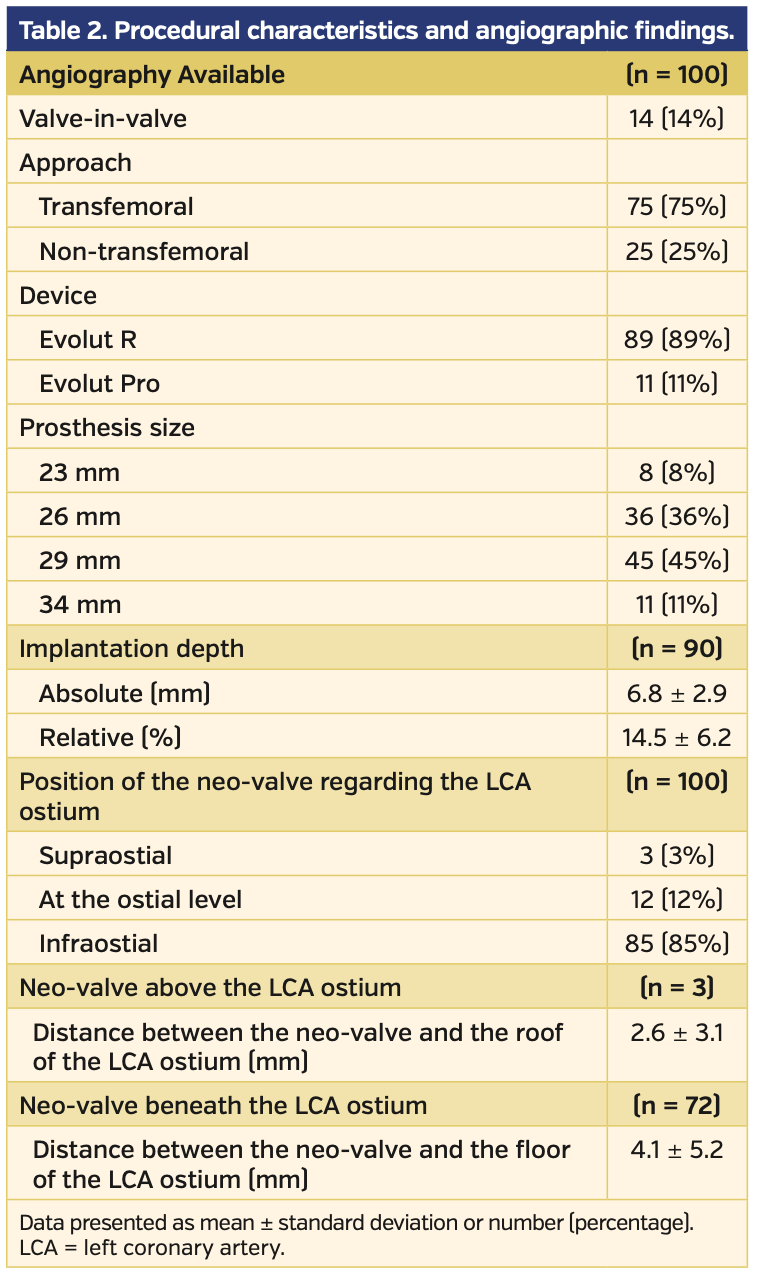
Angiographic analysis. The main baseline procedural characteristics and angiographic findings of the study population are summarized in Table 2. Most devices implanted were Evolut R valves (89%), and a valve-in-valve procedure was performed in 14% of patients. Measurement of the distance between the neo-valve and the floor of the LCA ostium (neo-valve in infraostial position, n = 85) and between the neo-valve and the roof of the LCA ostium (neo-valve in supraostial position, n = 3) was feasible in 85% and 100% of patients, respectively. An accurate assessment of the implantation depth was feasible in 90% of patients.
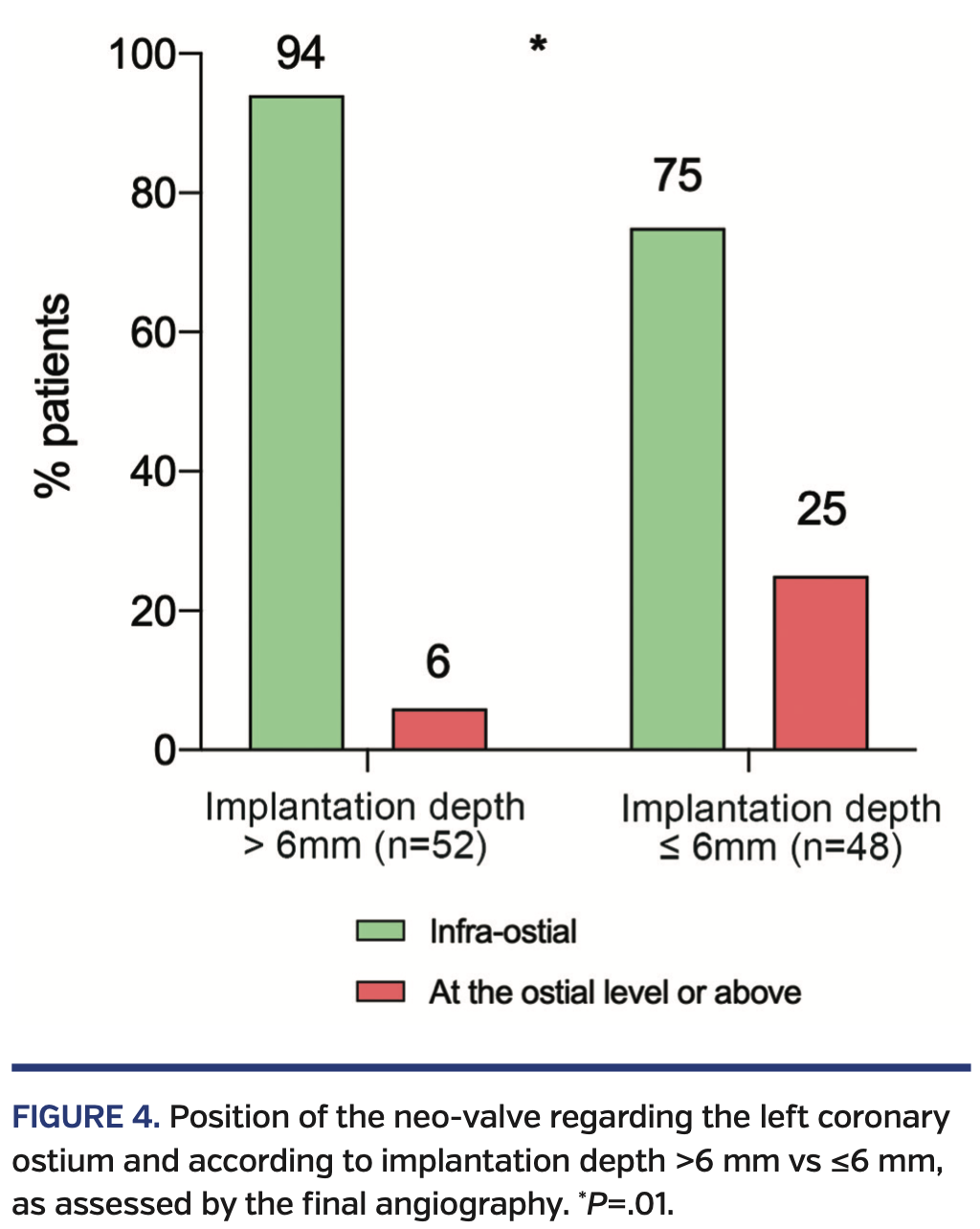
In regard to the LCA ostium, the neo-valve was in an infraostial position in 85% of cases, with a mean distance between the neo-valve and the floor of the LCA ostium of 4.1 ± 5.2 mm. The neo-valve was above the LCA ostium in 3% of cases and the mean distance between the neo-valve and the roof of the LCA ostium was 2.6 ± 3.1 mm. The median valve implantation depth was 6.45 mm (IQR, 4.83-8.75 mm), which is 15 ± 6% of the total device length. When comparing cases with implantation depth >6 mm and ≤6 mm, patients with implantation depth ≤6 mm exhibited a higher rate of neo-valve at or above the ostial level (25% vs 4% in patients with implantation depth >6 mm; P=.01) (Figure 4).
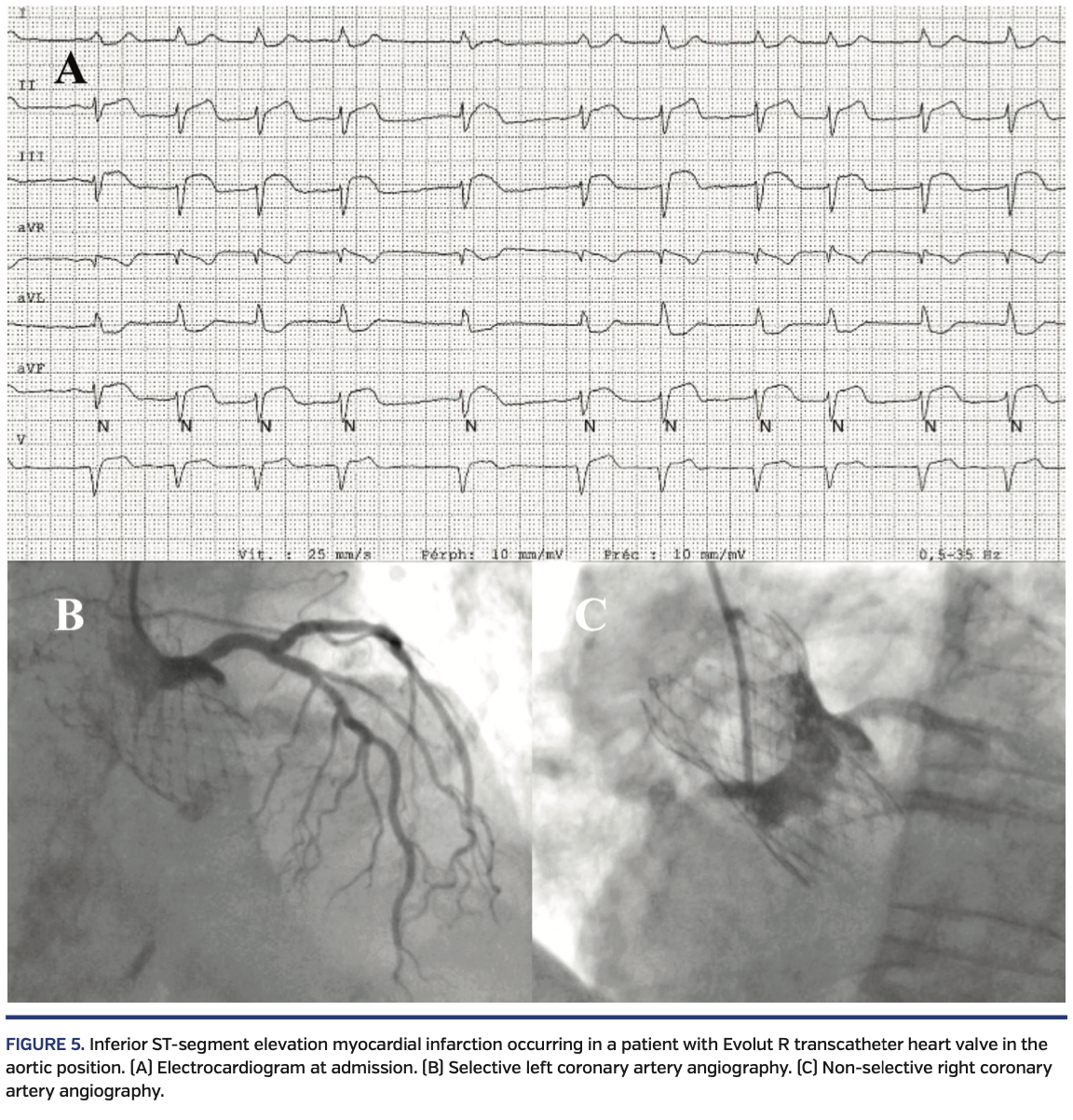
Coronary angiogram and PCI post TAVR. A total of 10 patients (10%) underwent coronary angiography and 2 patients (2%) underwent PCI at a mean of 12 ± 8 months post TAVR. Procedural characteristics of coronary angiography and PCI post TAVR are presented in Table 3. Most coronary angiograms (70%) were performed through radial approach. On average, 1.5 ± 0.5 and 2.1 ± 0.5 catheters were required to perform LCA and RCA injection, respectively. A selective injection of LCA and RCA was achieved in 60% and 40% of cases, respectively. Two attempts of coronary angioplasty were performed, and a failure occurred in 1 case. Briefly, a patient with prior Evolut R implantation was admitted for an inferior ST-segment elevation myocardial infarction 13 months post TAVR. The LCA could be selectively injected and was found to be free from significant coronary stenosis, and an acute thrombosis of the RCA with TIMI flow 0 was shown during a non-selective injection. However, the RCA revascularization was unsuccessful due to inability to selectively cannulate the RCA (Figure 4). A crossover from right radial artery access to femoral approach and a total of up to 6 different guiding catheters were used to attempt the cannulation of the RCA ostia. The patient was finally medically managed. Implantation depth was measured at 6.5 mm and the neo-valve was evaluated to be 1.7 mm underneath the LCA ostium, with the RCA ostium also shown to be above the neo-valve.
Discussion
The present study provides original data about the interaction between the neo-valve of the Evolut R/Pro system and the LCA ostium. The main findings of our study can be summarized as follows: (1) the neo-valve was supraostial in a minority of cases (3%); (2) the neo-valve was beneath the LCA ostium in 85% of patients, with a mean distance between the neo-valve and the LCA ostium floor of 4 mm; (3) an implantation depth ≤6 mm significantly increased the risk of the neo-valve positioning at or above the ostial level; and (4) coronary access issues leading to non-selective coronary injections were observed in a high percentage of patients requiring coronary angiography post TAVR.
Angiographic assessment. The long stent frame and supra-annular design of the Medtronic THV system explains why both LCA and RCA ostia are supposed to be covered in 100% of patients receiving such a device. Thus, post-TAVR coronary catheterization has to be performed through the struts of the THV in every case. However, the THV’s pericardial tissue interposition is inconstant and depends on the neo-valve level and the commissural post position with respect to the coronary ostium.5 In most cases (85%), the neo-valve was beneath the LCA ostium, with a mean distance of 4.1 mm between the neo-valve and the floor of the ostium. Nevertheless, a suspended commissure post that reaches 26 mm in height after positioning can be found in front of the coronary ostium in this configuration. Thus, a free coronary access (without valve tissue interposition) is not guaranteed, and the need to cross a lateral strut followed by a neo-sinus navigation is required in some cases. Conversely, the neo-valve was above the LCA ostium and at the ostial level in 3% and 12% of cases, respectively. In these cases, coronary re-access remains possible, but it imposes the need to cross a strut above the level of the ostium and then to navigate in the neo-sinus until achieving coronary catheterization. Tang et al7 recently described a technique regarding valve orientation at the time of implantation to reduce the severity of overlap of the THV commissures with one of the two coronary ostia, which may facilitate coronary access following Evolut R/Pro implant. Future studies are required to determine the reproducibility and broader application of this technique, along with its potential advantages regarding coronary access post TAVR.
Post-TAVR conduction disturbances, such as complete atrioventricular block leading to a permanent pacemaker implantation and new-onset persistent left bundle-branch block, are the most frequent complications of the procedure and remain more frequent with some self-expanding THV systems.3 Minimizing implantation depth allows a reduction in the rate of post-TAVR conduction disturbances,4 but are inevitably associated with an increase in THV/coronary ostia interaction. The higher rate of neo-valve at the ostial level or above when implantation depth is ≤6 mm highlights this relation. Also, coronary access is not guaranteed even when the neo-valve is underneath the coronary ostium level (mainly because of the suspended commissure post) and is likely to become very challenging when the neo-valve is at the ostium level or above. Thus, it is of paramount importance to consider both the possibility of conduction disturbances and coronary reaccess post TAVR (particularly in those patients with low coronary artery ostia) when trying to define an optimal THV position, especially as we expand TAVR indications to low-risk patients.
Coronary angiogram and PCI post TAVR. The reported rate of successful selective coronary angiogram after TAVR varies widely, ranging from 50%-100%.6 In the present study, a total of 10 patients underwent coronary angiography post TAVR. The rate of unselective injection was high (40% for the LCA and 60% for the RCA), with an average of 1.5 and 2.1 catheters required to perform LCA and RCA injections, respectively. Two coronary angioplasties were attempted, and PCI failure occurred in 1 case. This failure was related to an inability to adequately cannulate the RCA caused by the self-expanding THV. Interestingly, RCA cannulation was impossible despite the fact that the neo-valve was in an infraostial position, suggesting that the suspended commissural post was in front of the RCA ostium and therefore made the RCA catheterization impossible. This case emphasizes that catheterization of the RCA through a self-expanding Evolut valve system can be challenging, even when the THV is in the infraostial position.
Study limitations. The present study suffers from bias inherent to its single-center and retrospective design; thus, unmeasured confounders cannot be excluded. Neo-valve position in relation with RCA ostium was not assessed due to a frequent suboptimal opacification and to the fact that orthogonal view rarely allowed an acceptable view of the RCA ostium. Finally, the exact position of the suspended commissure post was not assessed, opening a new venue for dedicated studies with systematic post-TAVR electrocardiography-gated computed tomography angiography.
Conclusion
The Evolut R/Pro THV neo-valve was positioned below the LCA ostium in the vast majority (85%) of cases, but an implantation depth ≤6 mm was associated with a higher rate of neo-valve position at the coronary ostial level or above. Also, some difficulties in achieving adequate selective coronary injections post TAVR were observed in a high percentage of patients. These results should be considered in view of the current tendency toward very high (aortic) THV implants to avoid conduction disturbances, and future studies should determine the impact of Evolut THV position on potential coronary access issues post TAVR.
Acknowledgments. Dr Rodés-Cabau holds the Research Chair “Fondation Famille Jacques Larivière” for the Development of Structural Heart Disease Interventions.
*Joint first authors.
From the Quebec Heart and Lung Institute, Laval University, Quebec City, Quebec, Canada.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Faroux reports fellowship support from Institut Servier and the Association Régionale de Cardiologie de Champagne-Ardenne (ARCCA); research grant support from Biotronik, Edwards Lifesciences, and Medtronic. Dr Rodés-Cabau reports institutional research grants from Edwards Lifesciences, Medtronic, and Boston Scientific. The remaining authors report no conflicts of interest regarding the content herein.
Manuscript submitted November 28, 2019, final version accepted December 4, 2019.
Address for correspondence: Josep Rodés-Cabau, MD, Quebec Heart & Lung Institute, Laval University, 2725 Chemin Ste-Foy, G1V4G5, Quebec City, Quebec, Canada. Email: josep.rodes@criucpq.ulaval.ca
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695-1705.
- Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706-1715.
- Auffret V, Puri R, Urena M, et al. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation. 2017;136:1049-1069.
- Jilaihawi H, Zhao Z, Du R, et al. Minimizing permanent pacemaker following repositionable self-expanding transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12:1796-1807.
- Yudi MB, Sharma SK, Tang GHL, Kini A. Coronary angiography and percutaneous coronary intervention after transcatheter aortic valve replacement. J Am Coll Cardiol. 2018;71:1360-1378.
- Faroux L, Guimaraes L, Wintzer-Wehekind J, et al. Coronary artery disease and transcatheter aortic valve replacement: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:362-372.
- Tang GHL, Zaid S, Gupta E, et al. Impact of initial Evolut transcatheter aortic valve replacement deployment orientation on final valve orientation and coronary reaccess. Circ Cardiovasc Interv. 2019;12:e008044.