Impact of Rapid Ventricular Pacing During Transcatheter Implantation of Self-Expanding Aortic Valve Prostheses in Patients at Highest Risk
Abstract: Background. Patients with low-flow, low-gradient (LFLG) aortic stenosis (AS) have the highest risk among all AS patients. Rapid ventricular pacing (RVP) is frequently used during transcatheter aortic valve implantation (TAVI), but may negatively impact critical left ventricular function in these patients. We investigated the effects of RVP in patients with LFLG-AS undergoing implantation of a self-expandable valve prosthesis. Methods. In this retrospective study, patients with LFLG-AS were classified according to the number of cumulative RVP episodes. Results. Thirty-one patients with no episodes of RVP, 46 patients with 1 episode, and 40 patients with 2 or more episodes (2+) were identified. Society of Thoracic Surgeons (STS) scores in patients with 0, 1, or 2+ RVP episodes were 5.1% (interquartile range [IQR], 3.5%-8.9%), 6.0% (IQR, 3.8%-8.8%), and 4.8% (3.8%-8.1%), respectively. Peri-interventional adverse events tended to be highest in the group with 1 RVP. Residual aortic regurgitation was in 3.2%, 4.8%, and 2.6% (P=.87) in patients with 0, 1, and 2+ RVP episodes, respectively. Thirty-day mortality rates were 3.2%, 6.5%, and 7.5% (P=.74) and 1-year mortality rates were 22.6%, 30.4%, and 20.0% (P=.51) in patients with 0, 1, and 2+ RVP episodes, respectively. STS score, body mass index, prevalence of chronic lung disease, and more-than-mild residual aortic regurgitation emerged as independent predictors of 1-year mortality, whereas the number of RVP episodes had no impact on outcomes. Conclusions. One-year mortality is not influenced by RVP, but is influenced by the individual patient’s risk. The final implantation results in patients with 2+ RVP episodes suggest that RVP during implantation of self-expandable TAVI prostheses should not be withheld in an attempt to achieve optimal results, even in LFLG-AS patients.
J INVASIVE CARDIOL 2020;32(12):E355-E361. Epub 2020 November 22.
Key words: outcomes, TAVI, transcatheter heart valve
Rapid ventricular pacing (RVP) is one of the essential tools for transcatheter aortic valve implantation (TAVI) and is a prerequisite for implantation of balloon-expandable valves. RVP during TAVI leads to systemic hemodynamic depression1 and to reduced microvascular tissue perfusion;2 it is associated with an increase in troponin serum levels3 and transient depression of both systolic and diastolic left ventricular function.4 The clinical impact of these observations is not clear.3,5 Data on the role of RVP in outcomes are conflicting due to important confounding factors, such as transapical vs transfemoral access, the use of balloon-expandable vs self-expanding prostheses, and the lack of selection criteria related to patient risk, which may render them more or less vulnerable to RVP.6
We sought to investigate the effect of RVP in patients at highest risk. Patients with low-flow, low-gradient (LFLG) aortic stenosis (AS) by definition have not only reduced left ventricular ejection fraction and low stroke volume, but also a markedly higher cumulative prevalence of cardiovascular risk factors and manifest cardiac and non-cardiac diseases.7 Under these conditions, the TAVI operator often chooses the less harmful approach in these patients, namely, transfemoral access and implantation of self-expanding valves without the need for predilation, which precludes the need for RVP during TAVI.
Methods
Study population. This study was a retrospective analysis of data from a TAVI registry initiated in 2011 at a single high-volume center. All patients in this registry were referred for TAVI based on a local heart team decision. The approval for this study was obtained from the institutional ethics committee. Due to the retrospective nature of this study, a waiver of written informed consent was issued by the ethics committee. Only patients with severe LFLG-AS according to recent guidelines8 (aortic valve area indexed to body surface area <0.6 cm/m2; mean pressure gradient [MPG] <40 mm Hg; stroke volume index ≤35 mL/m2; left ventricular ejection fraction <50%) treated via the transfemoral approach with a self-expanding TAVI prosthesis were included. Individual episodes of RVP (all achieved by temporary pacemaker-induced right ventricular stimulation) were recorded cumulatively, taking into account any preimplantation episodes, any RVP during valve deployment, and any postimplantation pacing episodes. Patients were grouped as 0 RVP episodes during TAVI; 1 RVP episode before, during, or after TAVI implantation; or 2+ RVP episodes.
Follow-up visits and outcomes of interest. Follow-up visits were scheduled at 30 days and 1 year. Echocardiographic exams were scheduled before TAVI, before hospital discharge, and at the 30-day follow-up. The primary endpoint was death from any cause. Death of unknown origin was classified as cardiovascular. Patients with follow-up >1 year were censored as alive after 365 days.
Echocardiographic measurements. The left ventricular mass was calculated by the linear method.9 Left ventricular hypertrophy was defined as left ventricular mass >95 g/m2 in women and >115 g/m2 in men.9 The left ventricular ejection fraction was estimated visually. Stroke volume was determined at the left ventricular outflow tract by multiplying area by the systolic velocity integral and was indexed to body surface area. Aortic valve area was calculated according to the continuity equation.8
Measurement of aortic valve calcification. Non-contrast computed tomography was performed using a multidetector system (Somatom Definition or Force; Siemens Healthineers). Aortic valve calcium measurements were performed offline on dedicated workstations using validated software (syngo.via; Siemens Healthineers) by the Agatston method and are expressed in arbitrary units (AU).
Measurements of biomarkers. Venous blood samples were collected in ethylenediaminetetra-acetate-filled tubes for the determination of high-sensitivity cardiac troponin I (hs-cTnI) and creatine kinase MB (CK-MB) levels before and directly after TAVI, as well as at 4, 24, 48, and 72 hours after TAVI. Details of the methods have been reported elsewhere.10
Statistical analysis. Data are provided as median and interquartile range (IQR). Categorical variables are presented as numbers and percentages. Continuous values were compared with the Kruskal-Wallis test and categorical variables with the Chi-Squared test. Survival curves were constructed using Kaplan-Meier estimates and were compared with the log-rank test. Analysis of 1-year mortality was performed using Cox regression. All variables were tested for collinearity and were included in the Cox regression model if the variance inflation factor was <5. All statistical analyses were carried out using the SPSS statistical package, version 24 (IBM Corporation).
Results
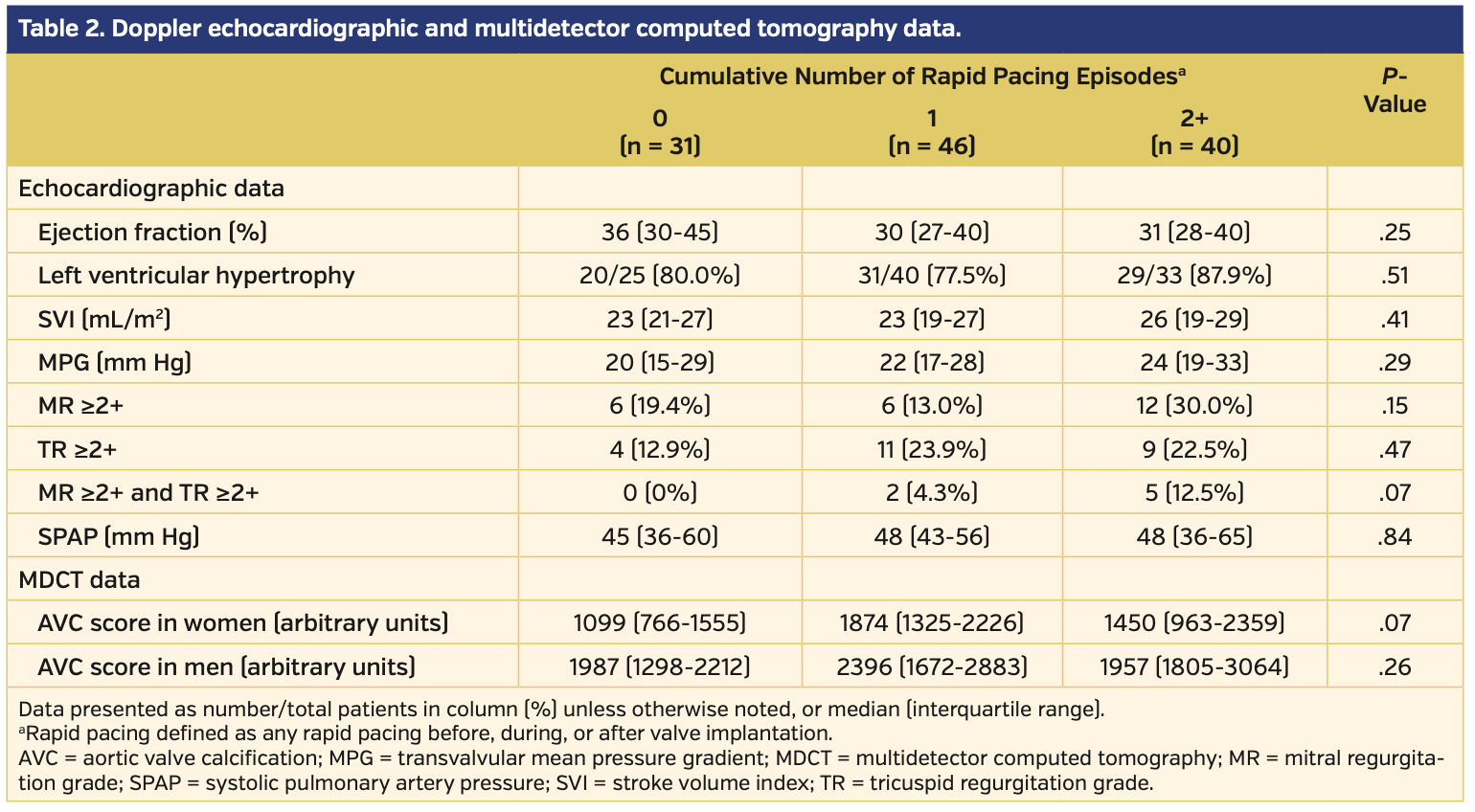
A total of 117 patients with LFLG-AS fulfilled the inclusion criteria. No RVP was performed in 31 patients, 1 RVP in 46 patients, and 2+ RVPs in 40 patients (2 RVPs in 32 patients and 3 RVPs in 8 patients) (Table 1). Of all instances of RVP use, it was applied during valve deployment in 10 patients. Age, sex, and distribution of functional New York Heart Association (NYHA) class were not different between the groups. Patients with 0 RVPs had the highest overall body mass index (BMI), and patients with 2+ RVPs had the lowest BMI (P=.03). The presence of cardiovascular risk factors or manifest cardiovascular diseases was similar in the 3 groups. Patients with 1 RVP had the highest prevalence of atrial fibrillation. Society of Thoracic Surgeons (STS) scores were 5.1% (IQR, 3.5%-8.9%), 6.0% (IQR, 3.8%-8.8%), and 4.8% (IQR, 3.8%-8.1%) in patients with 0, 1, or 2+ RVP episodes, respectively (P=.69). Median ejection fraction ranged between 30% and 36%, without differences between groups (Table 2). Mean stroke volume index and MPG were similar in the 3 groups. The prevalence of more-than-mild atrioventricular valve regurgitation was highest in patients with 2+ RVP episodes.
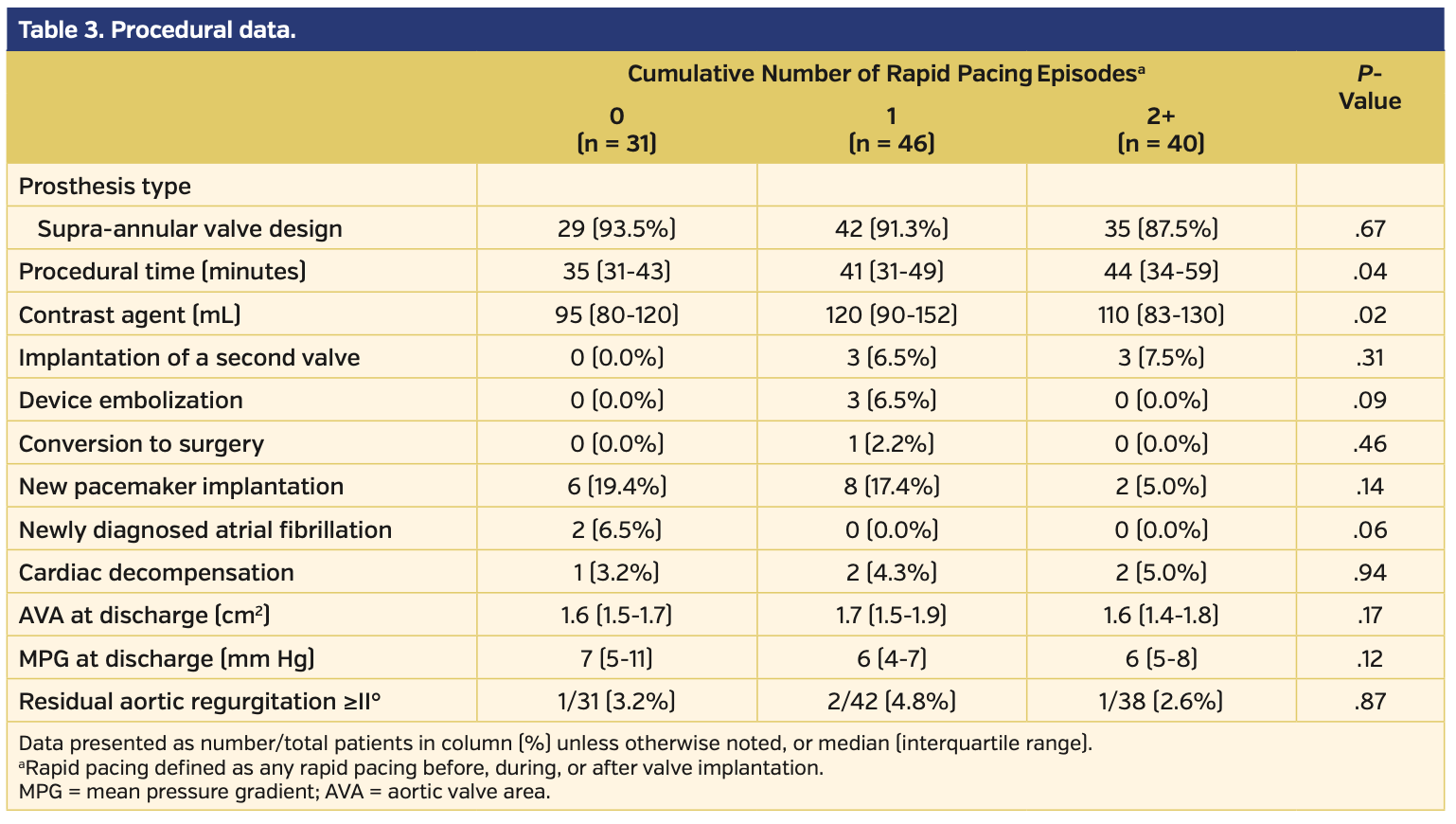
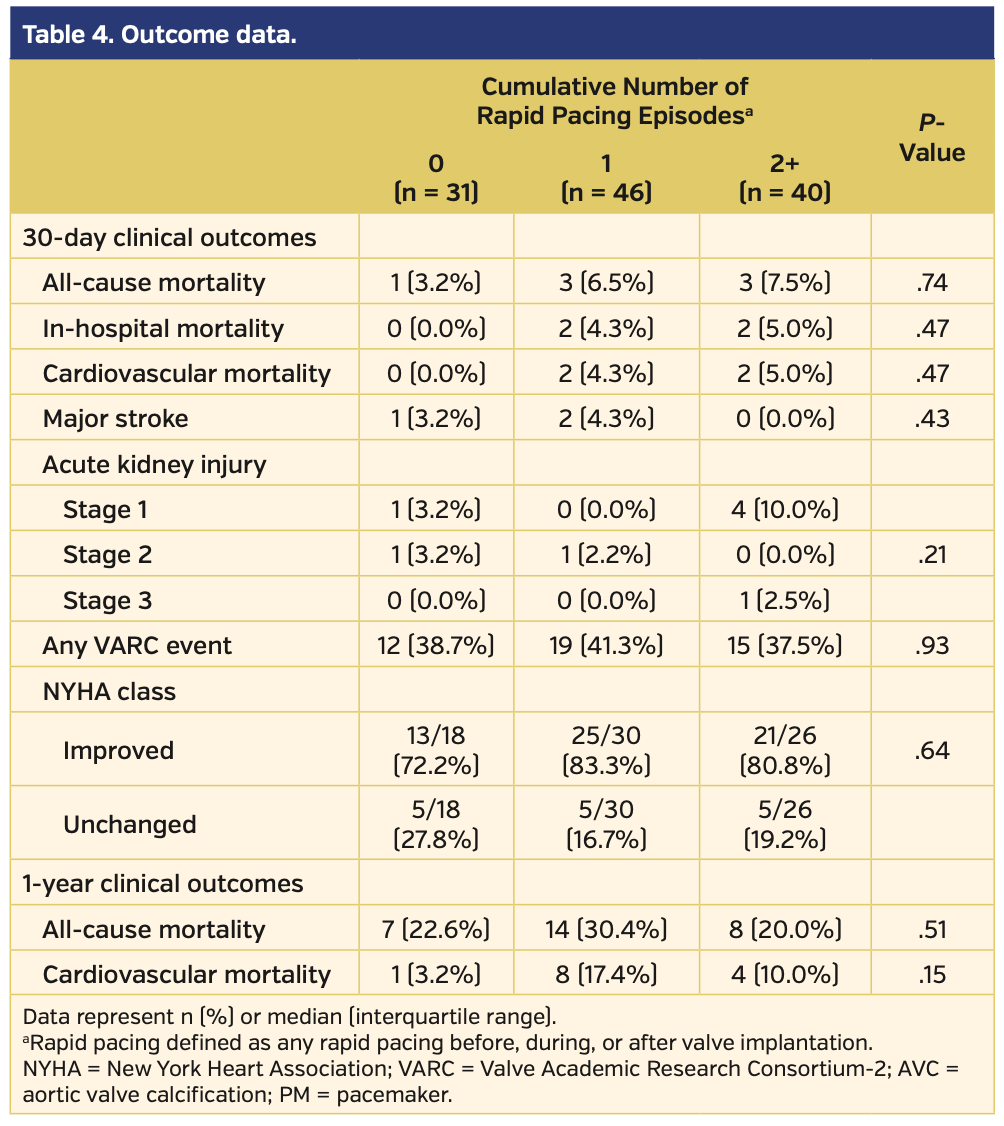
More than 88% of all patients were implanted with prostheses with a supra-annular valve design (Table 3). Procedural times increased from patients with 0 RVP to those with 1 or 2+ episodes of RVP (P=.04), and the amount of contrast agent was higher in patients with 1 or 2+ RVP episodes (P=.02). Intraprocedural adverse events, including implantation of a second valve, device embolizations, or conversion to surgery, occurred most often in patients with 1 RVP. New pacemaker implantation was slightly more frequent in patients with 0 or 1 RVP episode. Newly diagnosed atrial fibrillation was present in 2 patients in the 0 RVP group and no patients in the other groups. There was no difference between groups with respect to the frequency of cardiac decompensation after TAVI. The calculated aortic valve area and the MPG at discharge were identical between groups. Residual aortic regurgitation ≥II° was observed in 3.2%, 4.8%, and 2.6% in the groups with 0 RVP, 1 RVP, and 2+ RVP, respectively (P=.87). All-cause and cardiovascular mortality at 30 days tended to be lower in patients with 0 RVP and higher in those with 2+ RVPs, although these differences were not statistically significant (Table 4). The sum of cumulative events (as defined by Valve Academic Research Consortium [VARC]-2 criteria) was not different between groups. NYHA functional status improved in the majority of patients in all groups. All-cause mortality at 1-year follow-up was somewhat higher in patients with 1 RVP episode and lower in those patients with 2+ RVP episodes (Figure 1); however, possibly due to the small patient numbers, these changes were not significant.
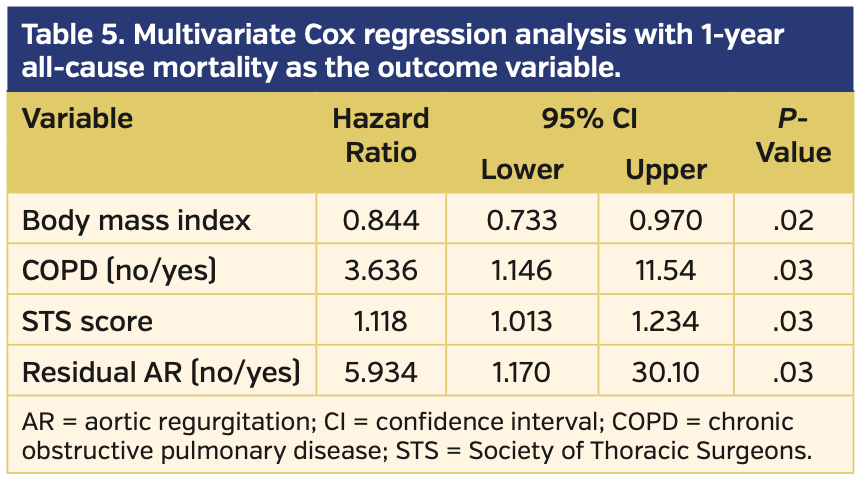
The impact of different variables on outcome in the 3 RVP groups was assessed by Cox regression analysis. Variables with significant or borderline significant differences between groups (BMI, glomerular filtration rate, arterial hypertension, chronic obstructive pulmonary disease, atrial fibrillation, mitral regurgitation > grade II and tricuspid regurgitation > grade II, aortic valve calcium, procedural time, amount of contrast agent) or with known prognostic impact (STS score, residual aortic regurgitation) were included together with RVP groups (Table 5). One-year all-cause mortality was predicted by a high STS score, a low BMI, and the prevalence of chronic obstructive pulmonary disease or more-than-mild residual aortic regurgitation.
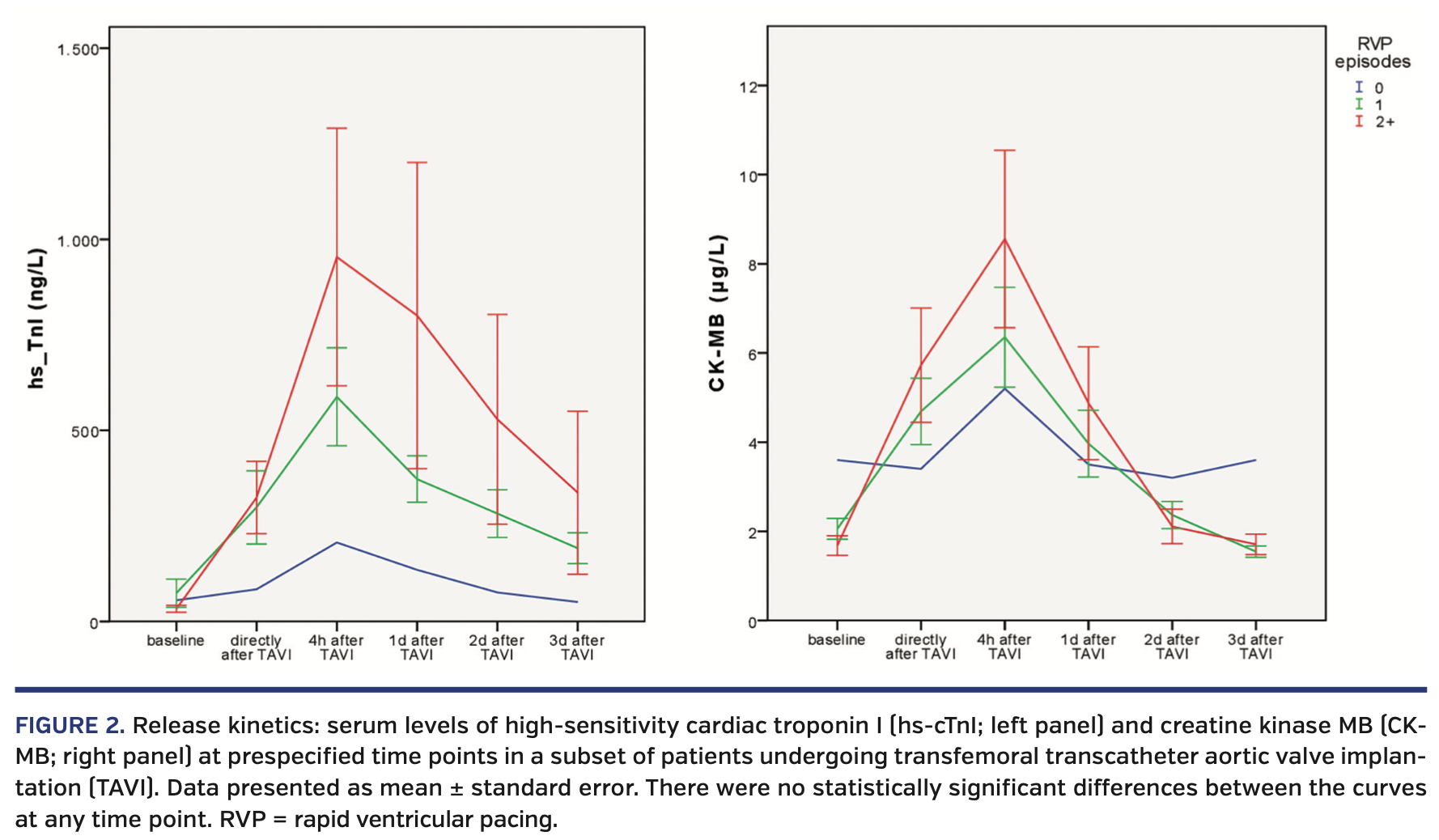
In a subset of 22 patients (1 patient with 0 RVP episodes, 9 patients with 1 RVP episode, and 11 patients with 2 RVP episodes), levels of the biomarkers hs-cTnI and CK-MB were measured serially prior to and up to 3 days after TAVI (Figure 2). Both myocardial markers showed an increase after TAVI, which was more pronounced in patients with 2 RVP episodes than in those with 0 or 1 RVP episodes. Differences between groups were not statistically different at any time point.
Discussion
Repeated RVP in patients with LFLG-AS during TAVI of self-expandable prostheses is associated with the release of myocardial markers and with a tendency for higher mortality during the first 30 days after implantation. However, the number of RVP episodes has no impact on 1-year mortality. Mortality at 1 year in the present study was determined solely by the given individual patient risk. The confirmation of the prognostic role of residual aortic regurgitation after TAVI may lead to the conclusion that RVP, if deemed necessary, should not be avoided during TAVI so that an optimal implantation result is achieved, even in patients at highest risk.
The novelty of our study is that we were able to analyze the effects of RVP in a well-defined, high-risk population. Thus, we avoided critical confounders such as those arising from the transapical approach, which leads to a release of myocardial markers,5,10,11 or the use of balloon-expandable valves, which makes RVP inevitable.3 Furthermore, the use of supra-annular valves was >90% in all groups, which becomes important when comparing postprocedural transvalvular gradients.
The application of RVP in our study was clearly associated with more complex TAVI procedures, as the procedural time and the amount of contrast agent were highest in groups with 1 RVP or 2+ RVP episodes. Interestingly, the highest rate of periprocedural adverse events occurred in the group with 1 RVP. A more profound interpretation of the short-term events is somewhat hampered by the small group size and the rather low number of adverse events. We did not observe a trend toward a higher prevalence of new atrial fibrillation3 or stroke3 in patients with increasing use of RVP. In fact, no major stroke was observed among patients with 2+ RVP episodes, and the cumulative rate of VARC-2 events after 30 days was identical among all groups. Likewise, our data did not indicate that hemodynamic responses were depressed in 2+ RVP patients, as there were no significantly higher rates of postimplant cardiac decompensation or acute kidney injury. There was a tendency for higher 30-day cardiovascular mortality with an increasing number of RVP episodes. However, while an association between RVP episodes and short-term mortality seems plausible, it is not meaningful to speculate about any causal relationship due to the very small sample sizes.
Findings concerning the long-term prognostic significance of RVP are controversial. In a recently published study on patients implanted with balloon-expandable and self-expanding valves,3 outcomes in patients with 1 to 2 RVP episodes (in 60% of all patients) were not different than in those with no RVP (in 13% of all patients), but ≥3 RVP episodes (in 27% of all patients) were associated with postimplantation acute kidney injury, a higher incidence of atrial fibrillation, and a higher 1-year mortality. However, patients in that study had a markedly lower risk than patients in our cohort, as reflected by 1-year mortality that varied between 7.7%-18% compared with mortality in our patients of 20.0%-30.4%. It is conceivable that parameters other than those assessed in our study may be of prognostic importance in the patient population at intermediate risk. Furthermore, the number of RVP episodes, and therefore the impact of RVP on outcomes, was greater in the other study due to the use of balloon-expandable valves,3 which intrinsically leads to a higher rate of RVP episodes. In contrast, the strength of our approach lies in the less frequent use of RVP or even the waiving of RVP during TAVI, which may represent a more up-to-date, straightforward TAVI procedure where RVP use is less pervasive.12,13
The consistently poor outcome of patients with LFLG-AS is usually associated with increased baseline cardiovascular risk and overt disease,7,14 and it seems that these baseline conditions bear a special impact on prognosis. The impact of BMI and chronic obstructive pulmonary disease on these patients has already been demonstrated.7 One can only speculate how implantation results may influence long-term outcomes in these patients. It is well known that residual aortic regurgitation is a predictor of adverse outcomes,15 especially in patients with heart failure,16 and it is conceivable that exposure of the vulnerable left ventricle of LFLG-AS patients to hemodynamic challenges like aortic regurgitation would negatively impact outcomes in these patients. Postdilation during RVP is often required to optimize implantation results and to reduce residual aortic regurgitation. Our results suggest that RVP should not be withheld in an attempt to achieve optimal implantation results, eg, a maximal reduction of residual aortic regurgitation, even in patients with critical left ventricular function.
Study limitations. Our study was a single-center, retrospective study with all the limitations inherent to such a study design. Selecting only high-risk patients led to rather small study populations, thereby limiting statistical significance of the findings. All of this may have affected the conclusions drawn from our study. We are not able to present data on the length of the RVP episodes, which may have altered the results. Echocardiographic measurements were made by different operators without a centralized core lab, and the ejection fraction was estimated visually. Finally, the data on myocardial marker release are incomplete, which makes it impossible to analyze the interplay between RVP, myocardial injury, and outcomes.
Conclusion
We observed that the long-term survival of high-risk patients with LFLG-AS after implantation of self-expanding aortic valve prostheses is affected primarily by the individual risk and not by the number of RVP episodes. Our confirmation of the prognostic significance of the implantation results in this high-risk population suggests that RVP, if necessary, should be applied even in LFLG-AS patients to achieve the best implantation results.
Acknowledgment. We thank Elizabeth Martinson, PhD, of the KHFI Editorial Office for excellent editorial work.
From the 1Department of Cardiology, Kerckhoff Heart Center, Bad Nauheim, Germany; 2Department of Cardiac Surgery, Kerckhoff Heart Center, Bad Nauheim, Germany; 3Medical Clinic I (Cardiology and Angiology), University Hospital of Giessen, Giessen, Germany; 4Department of Cardiology, Medical Clinic I, St. Johannes Hospital, Dortmund, Germany; and the 5German Centre for Cardiovascular Research (DZHK), Partner Site RhineMain, Bad Nauheim, Germany.
Disclosure: Dr Renker reports speaker fees from St. Jude Medical/Abbott Vascular. Dr Liebetrau reports speaker fees from Abbott Vascular. Dr Möllmann reports proctor/speaker fees from Abbott Vascular, Biotronik, Edwards Lifesciences, St. Jude Medical, and Symetis SA. Dr Hamm reports advisory board fees from Medtronic. Dr Kim reports proctor/speaker fees from Symetis, St. Jude Medical, and Edwards Lifesciences. Dr Fischer-Rasokat reports no conflicts of interest regarding the content herein.
Final version accepted April 29, 2020.
Address for correspondence: Ulrich Fischer-Rasokat, MD, Kerckhoff Heart Center, Benekestr. 2-8, 60231 Bad Nauheim, Germany. Email: u.fischer-rasokat@kerckhoff-klinik.de
- Iritakenishi T, Kamibayashi T, Torikai K, et al. Predictors of prolonged hemodynamic compromise after valve deployment during transcatheter aortic valve implantation. J Cardiothorac Vasc Anesth. 2015;29:868-874.
- Selle A, Figulla HR, Ferrari M, et al. Impact of rapid ventricular pacing during TAVI on microvascular tissue perfusion. Clin Res Cardiol. 2014;103:902-911.
- Fefer P, Bogdan A, Grossman Y, et al. Impact of rapid ventricular pacing on outcome after transcatheter aortic valve replacement. J Am Heart Assoc. 2018;7:e009038.
- Dworakowski R, Wendler O, Bhan A, et al. Successful transcatheter aortic valve implantation (TAVI) is associated with transient left ventricular dysfunction. Heart. 2012;98:1641-1646.
- Okitsu K, Iritakenishi T, Imada T, Iwasaki M, Shibata SC, Fujino Y. A longer total duration of rapid ventricular pacing does not increase the risk of postprocedural myocardial injury in patients who undergo transcatheter aortic valve implantation. Heart Vessels. 2017;32:1117-1122.
- Kapadia S, Agarwal S, Miller DC, et al. Insights into timing, risk factors, and outcomes of stroke and transient ischemic attack after transcatheter aortic valve replacement in the PARTNER trial (Placement of Aortic Transcatheter Valves). Circ Cardiovasc Interv. 2016;9:e002981.
- Fischer-Rasokat U, Renker M, Liebetrau C, et al. 1-year survival after TAVR of patients with low-flow, low-gradient and high-gradient aortic valve stenosis in matched study populations. JACC Cardiovasc Interv. 2019;12:752-763.
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739-2791.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233-270.
- Liebetrau C, Kim WK, Meyer A, et al. Identification of periprocedural myocardial infarction using a high-sensitivity troponin I assay in patients who underwent transcatheter aortic valve implantation. Am J Cardiol. 2017;120:1180-1186.
- Barbash IM, Dvir D, Ben-Dor I, et al. Impact of transapical aortic valve replacement on apical wall motion. J Am Soc Echocardiogr. 2013;26:255-260.
- Kim WK, Praz F, Blumenstein J, et al. Transfemoral aortic valve implantation of Edwards Sapien 3 without predilatation. Catheter Cardiovasc Interv. 2017;89:E38-E43.
- Schymik G, Rudolph T, Jacobshagen C, et al. Balloon-expandable transfemoral transcatheter aortic valve implantation with or without predilation: findings from the prospective EASE-IT TF multicentre registry. Open Heart. 2019;6:e001082.
- Conrotto F, D’Ascenzo F, D’Amico M, et al. Outcomes of patients with low-pressure aortic gradient undergoing transcatheter aortic valve implantation: a meta-analysis. Catheter Cardiovasc Interv. 2017;89:1100-1106.
- Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol. 2013;61:1585-1595.
- Urena M, Webb JG, Eltchaninoff H, et al. Late cardiac death in patients undergoing transcatheter aortic valve replacement: incidence and predictors of advanced heart failure and sudden cardiac death. J Am Coll Cardiol. 2015;65:437-448.