ADVERTISEMENT
Iatrogenic Aortopulmonary Window After Balloon Dilation of Left Pulmonary Artery Stenosis Following Arterial Switch Operation
ABSTRACT: Branch pulmonary artery stenosis may occur in 4%-28% of patients after an arterial switch operation. Balloon dilation can be attempted with variable results, while stenting is a more definitive option when balloon dilation fails. We report the case of a young boy who underwent balloon dilation of a stenosed left pulmonary artery 9 years after an arterial switch operation and was noted to have an aortopulmonary window about a year later. This was treated with covered stent implantation, which dealt both with the aortopulmonary window and the residual stenosis. The diagnostic process with cardiac magnetic resonance imaging and cardiac catheterization of such an unusual entity as well as the transcatheter management are discussed in detail.
J INVASIVE CARDIOL 2013;25(9):E188-E190
Key words: arterial switch operation, pulmonary artery stenosis
_____________________________
Transposition of great arteries is encountered in 1 in 3500-5000 live births. In 50% of cases, the ventriculo-arterial discordance is an isolated finding.1 In the early days of cardiac surgery, repair was achieved with the Senning or Mustard operations, whereby the venous blood was directed to the pulmonary artery (PA) by redirecting the flow at the atrial level.2 This was replaced by the arterial switch operation in the late 1970s to early 1980s.3-5 In this operation, the main PA is transected and the posteriorly located PAs are transferred anteriorly and placed like a “jockey on a horse” in front of the aorta. PA stenosis may develop in 4%-28% of children after the arterial switch operation.6-9 This is dealt with by transcatheter interventions, ie, either balloon angioplasty or stent placement in the stenosed branch.10-13 We report a rare complication of percutaneous intervention in this setting, whereby an aortopulmonary window developed between the anteriorly located left PA and posteriorly located ascending aorta, following balloon dilation of the left PA. The diagnostic process with cardiac magnetic resonance imaging (MRI) and catheterization as well as the interventional management with placement of a covered stent are discussed.
Case Report. Our patient presented with left PA stenosis following an arterial switch procedure that was performed at 30 days of age. The stenosis had become more severe with time and at the age of 9 years he underwent balloon angioplasty using gradually upsized balloons of 8 mm, 10 mm, and 12 mm. The procedure was uneventful and no technical difficulty was reported. However, at the end of the procedure, the left PA pressure rose to 110/67 mm Hg (equal to the aortic pressure) and the patient immediately suffered unilateral left-sided pulmonary edema. He was transferred to the intensive care unit and was successfully extubated 2 days post procedure. He was discharged home the next day.
The patient’s care was taken over by our team about a year later, when his family reported that he was not gaining weight, he had started becoming breathless on exercise, and a visible parasternal cardiac impulse had become evident. He was receiving captopril 3 times daily. On examination, the patient had a continuous murmur over the left subclavicular area and a loud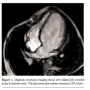 second heart sound. Echocardiogram showed a hugely dilated left ventricle (LV) of unknown cause (LV end-diastolic diameter of 60 mm; z-score, +8). There was mild tricuspid regurgitation with Doppler-derived gradient of 60 mm Hg. Imaging of the branch PAs and the arch was very difficult; hence, the patient was referred for MRI scan to investigate the unusual clinical and echocardiographic findings. Cardiac MRI confirmed severe LV dilation, with end-diastolic volume of 187 mL/m2 (Figure 1) and continuous flow in the left PA throughout systole and diastole, as detected with the phase-contrast flows taken in the left PA (Figure 2). Although the left PA appeared severely stenosed, the stroke volume to both PAs (differential flow) was equal. In addition, the cumulative flow to the PAs was double that of the flow in the main PA, implying that an additional source of flow beyond the PA bifurcation was present. The possibility of an iatrogenic aortopulmonary window was raised and the patient was taken to the catheterization laboratory in order to
second heart sound. Echocardiogram showed a hugely dilated left ventricle (LV) of unknown cause (LV end-diastolic diameter of 60 mm; z-score, +8). There was mild tricuspid regurgitation with Doppler-derived gradient of 60 mm Hg. Imaging of the branch PAs and the arch was very difficult; hence, the patient was referred for MRI scan to investigate the unusual clinical and echocardiographic findings. Cardiac MRI confirmed severe LV dilation, with end-diastolic volume of 187 mL/m2 (Figure 1) and continuous flow in the left PA throughout systole and diastole, as detected with the phase-contrast flows taken in the left PA (Figure 2). Although the left PA appeared severely stenosed, the stroke volume to both PAs (differential flow) was equal. In addition, the cumulative flow to the PAs was double that of the flow in the main PA, implying that an additional source of flow beyond the PA bifurcation was present. The possibility of an iatrogenic aortopulmonary window was raised and the patient was taken to the catheterization laboratory in order to further delineate the anatomy. Ascending aortic angiogram showed significant opacification of the PAs (Figure 3). The aortopulmonary window could be crossed easily from the PA into the aorta by advancing a wedge catheter with the balloon inflated; hence, its diameter was above 1 cm. It was decided not to balloon-size the defect, because of the possibility of further increasing its size. Therefore, a 34-mm long covered stent that was premounted on a 16-mm diameter balloon was chosen and implanted at the exact point of the window, sealing it off and at the same time relieving the left PA stenosis (Figures 4 and 5).
further delineate the anatomy. Ascending aortic angiogram showed significant opacification of the PAs (Figure 3). The aortopulmonary window could be crossed easily from the PA into the aorta by advancing a wedge catheter with the balloon inflated; hence, its diameter was above 1 cm. It was decided not to balloon-size the defect, because of the possibility of further increasing its size. Therefore, a 34-mm long covered stent that was premounted on a 16-mm diameter balloon was chosen and implanted at the exact point of the window, sealing it off and at the same time relieving the left PA stenosis (Figures 4 and 5).
The patient improved after the stent implantation and was discharged home the following day.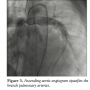 His medication was discontinued. His clinical progress was excellent and he started gaining weight. Cardiac MRI 14 months after the percutaneous procedure showed significant reduction of the LV dimensions from 187 to 117 mL/m2 (Figure 6). In addition, the left PA was widely patient and the flow pattern in it had now normalized and was no longer biphasic. The patient remains well and medication free 2 years after the procedure.
His medication was discontinued. His clinical progress was excellent and he started gaining weight. Cardiac MRI 14 months after the percutaneous procedure showed significant reduction of the LV dimensions from 187 to 117 mL/m2 (Figure 6). In addition, the left PA was widely patient and the flow pattern in it had now normalized and was no longer biphasic. The patient remains well and medication free 2 years after the procedure.
Discussion. The Lecompte procedure employed during the arterial switch operation involves transferring the PA branches directly in front of the aorta. There is a known risk of postoperative branch PA stenosis in about 4%-28% of patients.6-9 Balloon angioplasty of the branches has been reported with variable results,10-12 but may be effective in up to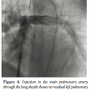 50% of patients, when the procedure takes place soon after the operation and particularly at <3.5 years old.13 In young and small patients, angioplasty, as opposed to stent implantation, may be attempted first. However, balloon angioplasty of PA stenosis in this setting has very rarely resulted in an iatrogenic aortopulmonary window.14-16 In the above 3 reports found in the English literature, 4 patients suffered an aortopulmonary communication as a result of PA balloon dilation post switch operation and were diagnosed either echocardiographically or by cardiac catheterization. Two of those patients were treated surgically and 2 underwent covered stent implantation.
50% of patients, when the procedure takes place soon after the operation and particularly at <3.5 years old.13 In young and small patients, angioplasty, as opposed to stent implantation, may be attempted first. However, balloon angioplasty of PA stenosis in this setting has very rarely resulted in an iatrogenic aortopulmonary window.14-16 In the above 3 reports found in the English literature, 4 patients suffered an aortopulmonary communication as a result of PA balloon dilation post switch operation and were diagnosed either echocardiographically or by cardiac catheterization. Two of those patients were treated surgically and 2 underwent covered stent implantation.
In our patient, the acute change in the recorded left PA pressure after balloon dilation, the development of unilateral pulmonary edema, as well as the subsequent long-term clinical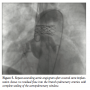 deterioration of the patient with severe LV dilation, pointed to the immediate development of the aortopulmonary window in the catheterization laboratory. This went unnoticed until his family noted a visually prominent cardiac impulse and severe LV dilation was found on echocardiogram. That finding, along with the continuous murmur on auscultation, pointed immediately to a left-to-right shunt. However, the echocardiogram, as is often the case for these patients, provided very poor-quality images of the great vessels. The cardiac MRI was therefore essential in order to delineate the anatomy and point toward the cause of the LV dilation. The MRI hint that pointed us to the diagnosis was the continuous flow in the left PA pattern as observed on the phase contrast images (Figure 2), along with the cumulative flow to the branch PAs, which was double that of the main PA, indicating an additional source of flow to the lungs. The suspicion was confirmed with cardiac catheterization and the window was sealed off with a covered stent.
deterioration of the patient with severe LV dilation, pointed to the immediate development of the aortopulmonary window in the catheterization laboratory. This went unnoticed until his family noted a visually prominent cardiac impulse and severe LV dilation was found on echocardiogram. That finding, along with the continuous murmur on auscultation, pointed immediately to a left-to-right shunt. However, the echocardiogram, as is often the case for these patients, provided very poor-quality images of the great vessels. The cardiac MRI was therefore essential in order to delineate the anatomy and point toward the cause of the LV dilation. The MRI hint that pointed us to the diagnosis was the continuous flow in the left PA pattern as observed on the phase contrast images (Figure 2), along with the cumulative flow to the branch PAs, which was double that of the main PA, indicating an additional source of flow to the lungs. The suspicion was confirmed with cardiac catheterization and the window was sealed off with a covered stent.
Conclusion. Although complications from balloon angioplasty or stent implantation in the branch PAs after the arterial switch procedure are rare, extra care should be taken regarding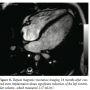 formation of tears and aortopulmonary communications, due to the proximity of the aorta to the branches. Covered stents may be used to treat the iatrogenic aortopulmonary windows in this setting. Cardiac MRI is very useful to either raise the suspicion or make the diagnosis, as it provides useful information on the ventricular volumes, flows, and anatomy, thus providing a solution to diagnostic puzzles, while guiding management.
formation of tears and aortopulmonary communications, due to the proximity of the aorta to the branches. Covered stents may be used to treat the iatrogenic aortopulmonary windows in this setting. Cardiac MRI is very useful to either raise the suspicion or make the diagnosis, as it provides useful information on the ventricular volumes, flows, and anatomy, thus providing a solution to diagnostic puzzles, while guiding management.
Acknowledgment. We wish to thank Dr Dimitra Loggitsi from Mitera Children’s Hospital and Dr Aaron Bell from Evelina Children’s Hospital for their input on the MRI diagnosis of this patient.
References
- Martins P, Castela E. Transposition of the great arteries. Orphanet J Rare Dis. 2008;3:27.
- Moons P, Gewillig M, Sluysmans T, et al. Long-term outcome up to 30 years after the Mustard or Senning operation: a nationwide multicenter study in Belgium. Heart. 2004;90(3):307-313.
- Yacoub MH, Radley-Smith R, Hilton CJ. Anatomical correction of complete transposition of the great arteries and ventricular septal defect in infancy. Br Med J. 1976;1(6018):1112-1114.
- Lecompte Y, Zannini L, Hazan E, et al. Anatomic correction of transposition of the great arteries. J Thorac Cardiovasc Surg. 1981;82(4):629-631.
- Idriss FS, Ilbawi MN, DeLeon SY, et al. Arterial switch in simple and complex transposition of the great arteries. J Thorac Cardiovasc Surg. 1988;95(1):29-36.
- Losay J, Touchot A, Serraf A, et al. Late outcome after arterial switch operation for transposition of the great arteries. Circulation. 2001;104(12Suppl1):121-126.
- Wernovsky G, Hougen TJ, Walsh EP, et al. Midterm results after the arterial switch operation for transposition of the great arteries with intact ventricular septum: clinical, hemodynamic, echocardiographic, and electrophysiologic data. Circulation. 1988;77(6):1333-1344.
- Lupinetti FM, Bove EL, Minich LL, et al. Intermediate-term survival and functional results after arterial repair for transposition of the great arteries. J Thorac Cardiovasc Surg. 1992;103(3):421-427.
- Yamaguchi M, Hosokawa Y, Imai Y, et al. Early and midterm results of the arterial switch operation for transposition of the great arteries in Japan. J Thorac Cardiovasc Surg. 1990;100(2):261-269.
- Zeevi B, Keane JF, Perry SB, Lock JE. Balloon dilation of postoperative right ventricular outflow obstructions. J Am Coll Cardiol. 1989;14(2):401-408.
- Saxena A, Fong LV, Ogilvie BC, Keeton BR. Use of balloon dilatation to treat supravalvar pulmonary stenosis developing after anatomical correction for complete transposition. Br Heart J. 1990;64(2):151-155.
- Formigari R, Santoro G, Guccione P, et al. Treatment of pulmonary artery stenosis after arterial switch operation: stent implantation vs. balloon angioplasty. Catheter Cardiovasc Interv. 2000;50(2):207-211.
- Nakanishi T, Matsumoto Y, Seguchi M, Nakazawa M, Imai Y, Momma K. Balloon angioplasty for postoperative pulmonary artery stenosis in transposition of the great arteries. J Am Coll Cardiol. 1993;22(3):859-866.
- Preminger TJ, Lock JE, Perry SB. Traumatic aortopulmonary window as a complication of pulmonary artery balloon angioplasty: transcatheter occlusion with a covered stent. A case report. Cathet Cardiovasc Diagn. 1994;31(4):286-289.
- Takayama H, Sekiguchi A, Chikada M, Noma M, Ishida R. Aortopulmonary window due to balloon angioplasty after arterial switch operation. Ann Thorac Surg. 2002;73(2):659-661.
- Vida VL, Biffanti R, Stellin G, Milanesi O. Iatrogenic aortopulmonary fistula occurring after pulmonary artery balloon angioplasty: a word of caution. Pediatr Cardiol. 2013;34(5):1267-1268. 2012 May 29 (Epub ahead of print).











