In-Hospital and 30-Day Mortality After Percutaneous Coronary Intervention in England in the Pre-COVID and COVID Eras
Abstract
Background. Public reporting of percutaneous coronary intervention (PCI) outcomes is a performance metric and a requirement in many healthcare systems. There are inconsistent data on the causes of death after PCI, and the proportion of these deaths that are attributable to cardiac causes. Methods. All patients undergoing PCI in England between January 1, 2017 and May 10, 2020 (n = 273,141) were retrospectively analyzed according to their outcome from the date of PCI: no death, in-hospital death, postdischarge death, and total 30-day death. The present study examined short-term primary causes of death after PCI in a national cohort before and during COVID-19. Results. The overall rates of in-hospital and 30-day death were 1.9% and 2.8%, respectively. The rate of 30-day death declined between 2017 (2.9%) and February 2020 (2.5%), mainly due to lower in-hospital death (2.1% vs 1.5%), before rising again from March 1, 2020 (3.2%) due to higher rates of postdischarge mortality. Only 59.6% of 30-day deaths were due to cardiac causes, with the most common causes being acute coronary syndrome, cardiogenic shock, and heart failure, and this persisted throughout the study period. In the 30-day death group, 10.4% after March 1, 2020 were due to confirmed COVID-19. Conclusions. In this nationwide study, we show that 40% of 30-day deaths are due to non-cardiac causes. Non-cardiac deaths have increased even more from the start of the COVID-19 pandemic, with 1 in 10 deaths from March 2020 being COVID-19 related. These findings raise a question of whether public reporting of PCI outcomes should be cause specific.
J INVASIVE CARDIOL 2021;33(3):E206-E219. Epub 2020 December 22. doi:10.25270/jic/20.00639
Key words: COVID-19, deaths, England, outcomes, percutaneous coronary intervention, SARS-coV-2
Percutaneous coronary intervention (PCI) is the most common modality for coronary revascularization worldwide.1,2 Advances in PCI techniques, stent platforms, and operator experience have all led to a reduction in mortality following PCI in recent years.3-5 Mortality post PCI may be attributed to both cardiovascular (CV) causes and non-CV causes, although PCI mortality risk-prediction models are focused on overall mortality.6-8 This is particularly relevant in the context of public reporting of operator PCI outcomes, which is often perceived as a surrogate of operator skill and quality of healthcare provided,9-14 even though 30-day mortality post PCI may not be directly related to the procedure. There are inconsistent findings among current data on the causes of death following PCI, with some studies suggesting that the majority of short-term mortality after PCI is CV in origin, while others show that CV mortality represents a minority of all deaths.7,15-17 However, the evidence to date is based on single- or multicenter registry analyses, older procedural cohorts (eg, pre 2010) and analyses from highly selected patient groups (eg, randomized controlled trials [RCTs]) that may not be generalizable to a contemporary PCI population. For example, a study of 115,191 patients undergoing PCI in the Veterans Affairs (VA) healthcare system showed that only a minority of deaths within 30 days (28%) were cardiac in origin.16 However, they analyzed a selected cohort comprising elderly males (median age, 71 years; 99% males) that is not representative of national PCI practice across different healthcare systems. In contrast, in an analysis of 21 RCTs, CV mortality was at least 5-fold higher than non-CV mortality (0.5% vs 0.1%, respectively) at 30 days.7
Data around changes in the cause of death from an all-comer national perspective following PCI are limited, particularly around the COVID-19 pandemic, which has infected more than 10 million patients worldwide, with the United Kingdom (UK) ranking second globally in terms of mortality.18 Recent studies have demonstrated higher COVID-19 related mortality in patients with CV disease.19-21 The underlying mechanisms behind increased COVID-19 mortality in patients with CV disease remain unclear, with possible explanations including factors such as advanced age, lower angiotensin-converting enzyme (ACE)-2 levels, and impaired immunity.22 Little is known about the characteristics, as well as rates and causes of death, of patients undergoing PCI in the current COVID era, and how these compare with those before the pandemic. The present study was designed to examine national-level trends and causes of death, up to 30 days post procedure, in a contemporary PCI cohort in England before and during the COVID-19 pandemic.
Methods
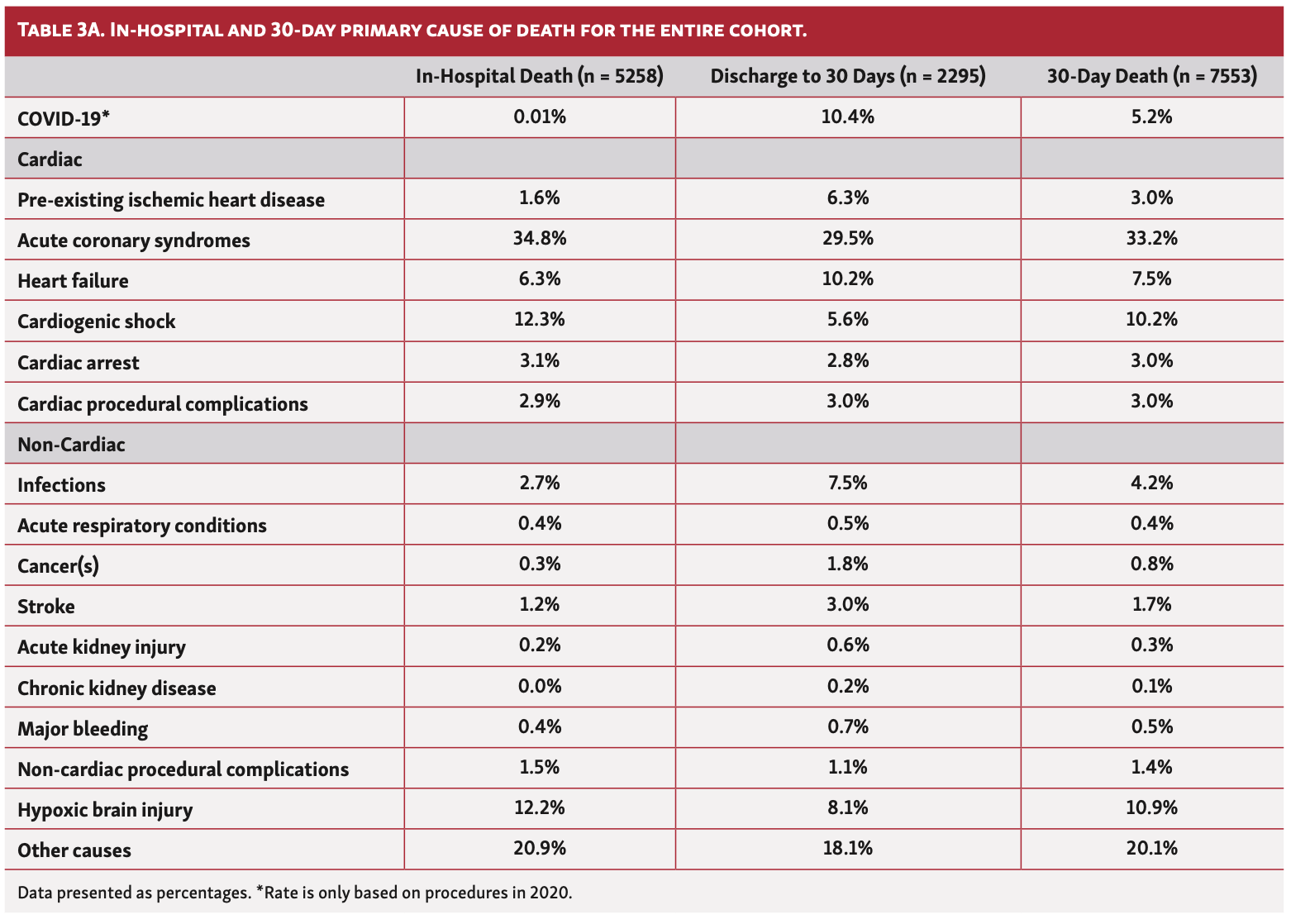
Data source, study design, and population. All adults (aged ≥18 years) undergoing PCI between January 1, 2017 and May 10, 2020 in England were retrospectively analyzed from the British Cardiovascular Intervention Society (BCIS) registry, and stratified by outcome into 4 groups according to outcome from the date of PCI: no death, in-hospital death, up to 30-day death post discharge (excluding in-hospital death), and 30-day total death. There were no specified inclusion or exclusion criteria except missing data on death (n = 299), deaths occurring >30 days post PCI (n = 12,220), and multiple PCI procedures for the same patient (n = 13,693), in which case only the final procedure was included with all other previous procedures were excluded (Figure 1). The BCIS registry, officially known as the National Audit of Percutaneous Coronary Intervention, comprises clinical, procedural, and in-hospital outcomes data for all procedures undertaken in the UK. 23,24 It is managed by the National Institute for Cardiovascular Research (NICOR), and commissioned by the Healthcare Quality Improvement Partnership (HQIP). Mortality beyond the in-hospital phase was collected via record linkage with the Office for National Statistics (ONS) Civil Registrations of Death dataset (up to the date of June 9, 2020).25 The process of death certification and registration is a legal requirement in the UK, where a doctor who has seen the deceased within the last 14 days of life must complete a Medical Cause of Death Certificate unless a post mortem examination is planned. International Classification of Diseases, tenth revision (ICD-10) codes were used to extract data on the most prevalent primary causes of deaths (first line on the death certificate), including COVID-19, from the ONS Civil Registrations of Death dataset. A full list of the diagnosis codes used in the study is provided Supplemental Table S1. Cardiac deaths included any death due to the following: acute coronary syndrome (ACS), heart failure, cardiogenic shock, cardiac arrest, pre-existing ischemic heart disease (IHD), and cardiac procedural complications.
Outcomes. The main outcome was post-PCI death at specific time points: in-hospital, up to 30 days post discharge, and total at 30 days post discharge, stratified into cardiac, non-cardiac, and COVID-19 related deaths.
Statistical analysis. For exploratory analysis, we examined patient and procedural characteristics of patients undergoing PCI according to their final outcome: no death, in-hospital death, postdischarge death up to 30 days, and total deaths at 30 days. Further comparisons were performed according to individual year, as well as pre- and post-COVID-19 pandemic in 2020 (procedures performed between January 1, 2020 to February 29, 2020, and March 1, 2020 to May 10, 2020, respectively), and clinical syndrome (stable angina vs ACS). Age was normally distributed and therefore summarized using mean ± standard deviation and compared using the t-test. Categorical variables were summarized as percentages and analyzed using the Chi-square test or Fisher’s exact test, where appropriate, and using the Kruskal-Wallis test for ordinal variables. Statistical analyses were performed using Stata 16 MP. Multivariable logistic regression models were performed to examine predictors of cardiac, non-cardiac, and COVID-related deaths, adjusting for the patient characteristics and procedural characteristics (only for cardiac and non-cardiac deaths) summarized in Appendix A, and are expressed as odds ratio (OR) with corresponding 95% confidence interval (CI). Multiple imputation with chained equations was performed for variables with missing data prior to model fitting, with a total of 10 imputations. Model estimates were later combined using Rubin’s rules.26 The frequency of missing data prior to imputation is provided in Supplemental Table S2.
Ethical approval. The UK Secretary of State for Health and Social Care has issued a time-limited Notice under Regulation 3(4) of the NHS (Control of Patient Information Regulations) 2002 (COPI) to share confidential patient information. The study complies with the Declaration of Helsinki. This work was part of a work stream endorsed by the Scientific Advisory Group for Emergencies (SAGE), the body responsible for ensuring timely and coordinated scientific advice is made available to UK government decision makers (SAGE supports UK cross-government decisions in the Cabinet Office Briefing Room [COBR]), and by NHS England, which overseas commissioning decisions in the NHS, and NHS Improvement, which is responsible for overseeing quality of care in NHS hospitals.
Results
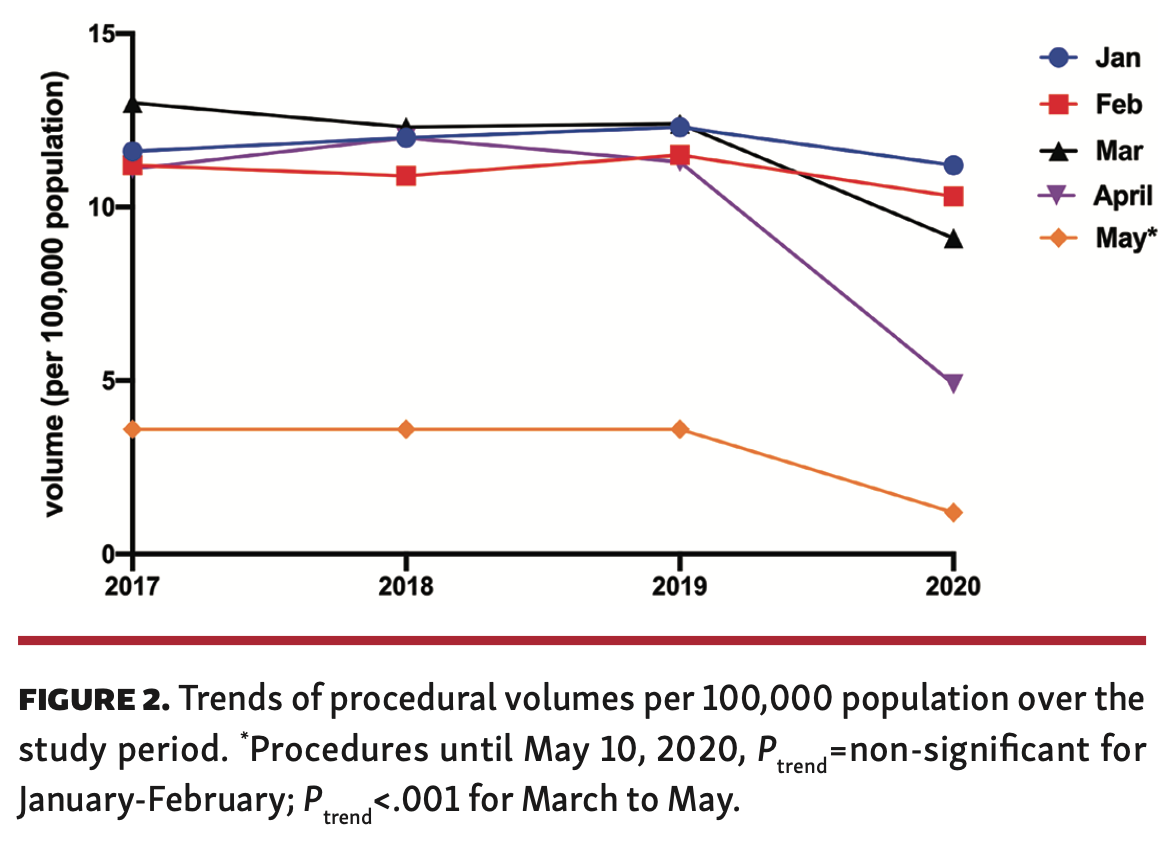
A total of 273,141 patients underwent PCI between January 1, 2017 and May 10, 2020. Overall, procedural volumes were similar between 2017 to 2019 (~83,000/year). The number of procedures per 100,000 population sharply declined between March 1, 2020 to May 10, 2020 compared with the same period in previous years (Figure 3), but was largely similar in January and February across all years.
30-Day Death Rates
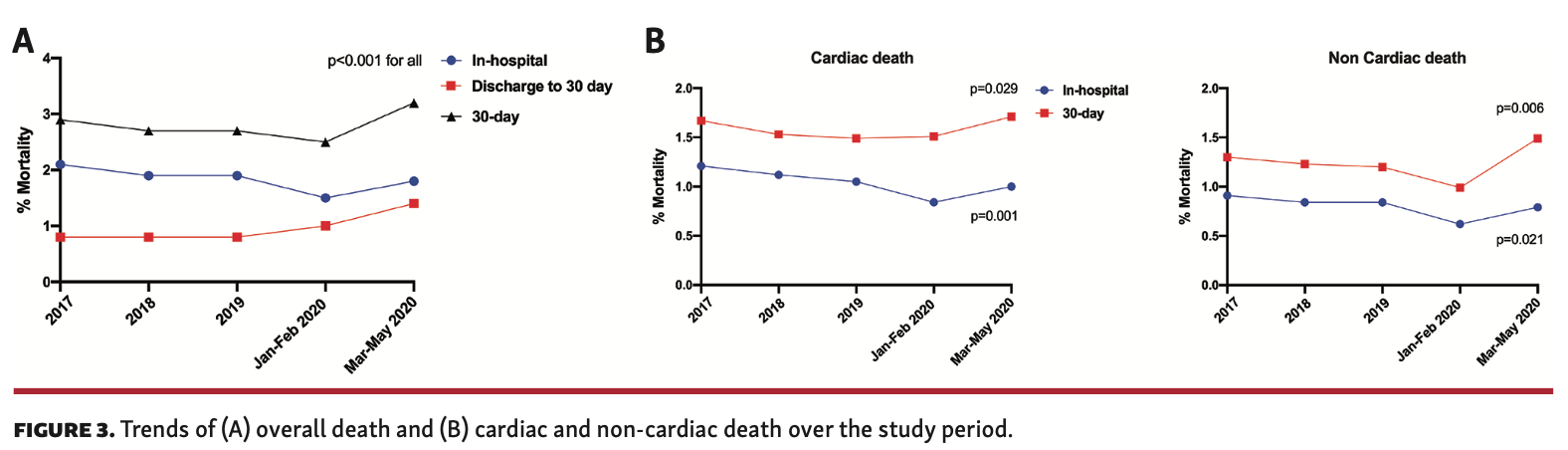
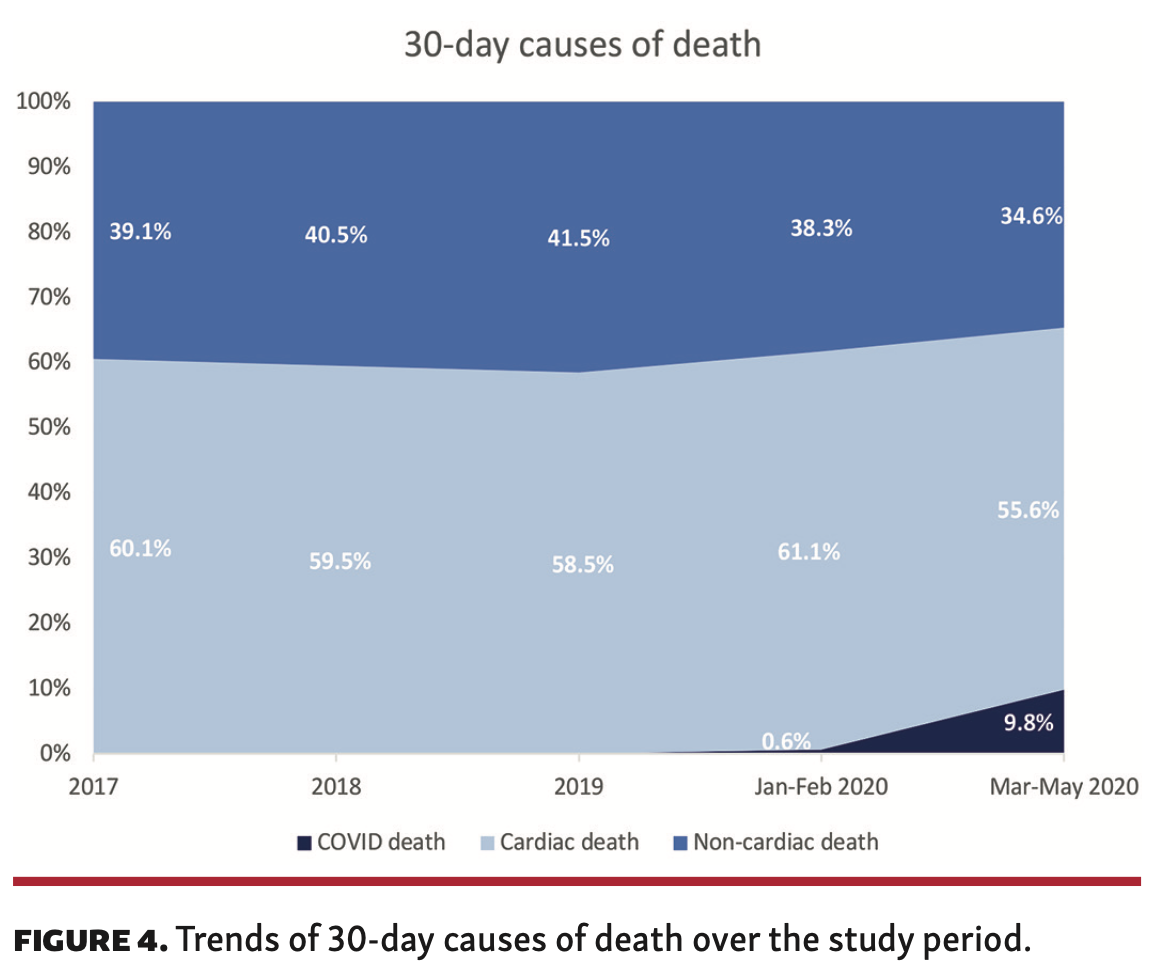
The rate of 30-day death in the overall cohort was 2.8% (n = 7553), the majority of which occurred in hospital (1.9%; n = 5258). The rate of 30-day death declined between 2017 and February 2020 (2.9% to 2.5%; P<.001), primarily driven by lower in-hospital death (2.1% vs 1.5%), before rising again between March 1, 2020 and May 10, 2020 (3.2%) due to higher rates of postdischarge mortality up to 30 days (Figure 3A). Overall, both in-hospital cardiac and non-cardiac death rates declined over the study period (2017 to May 2020: cardiac, 1.21% vs 1.00%; non-cardiac, 0.91% vs 0.79%). Overall, 59.7% (n = 4499) of 30-day deaths were due to cardiac causes. While 30-day cardiac and non-cardiac death rates both dropped between 2017 and February 2020, they were significantly increased in patients undergoing PCI between March 1, 2020 and May 10, 2020 (Figure 3B).
Patient and Procedural Characteristics
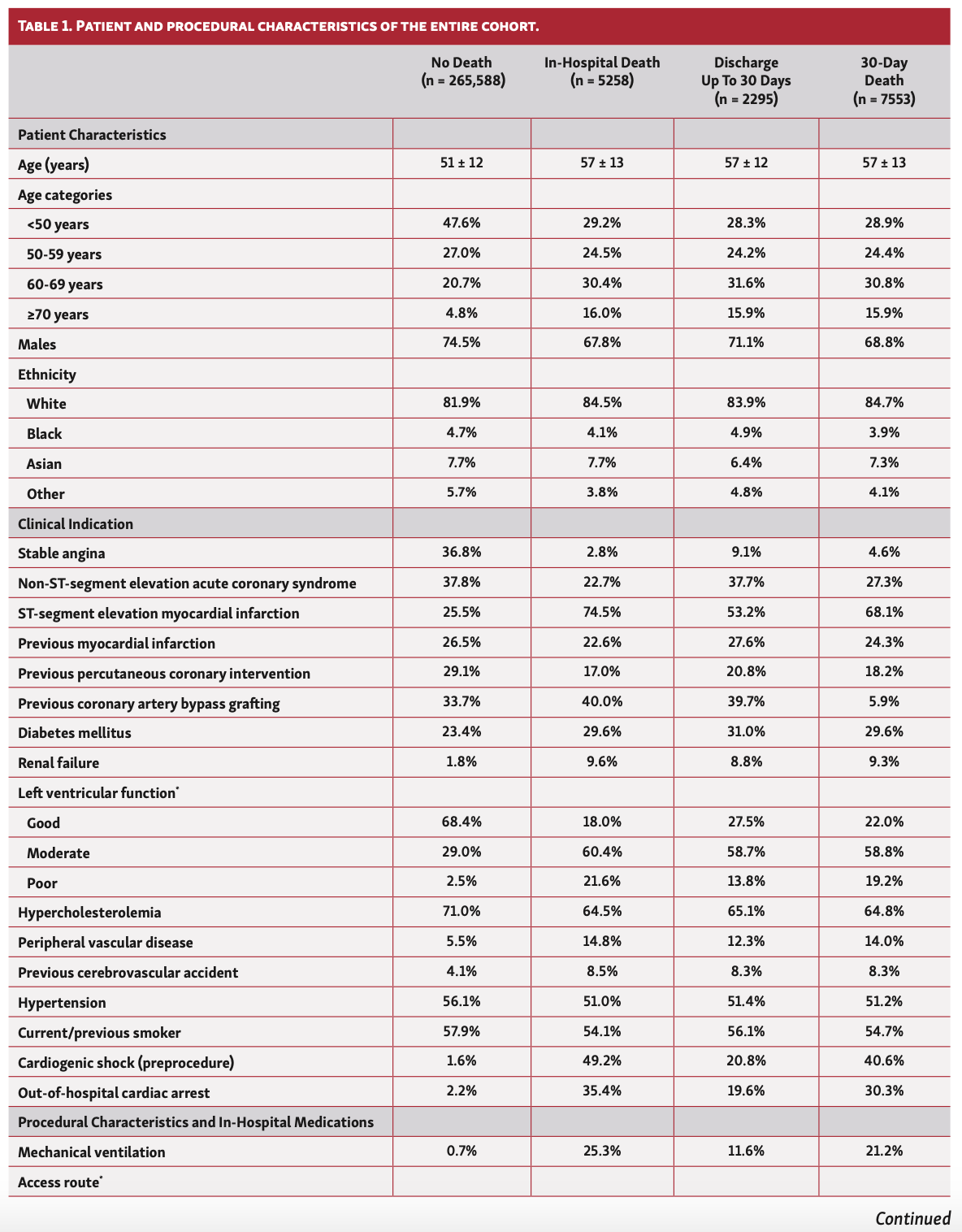
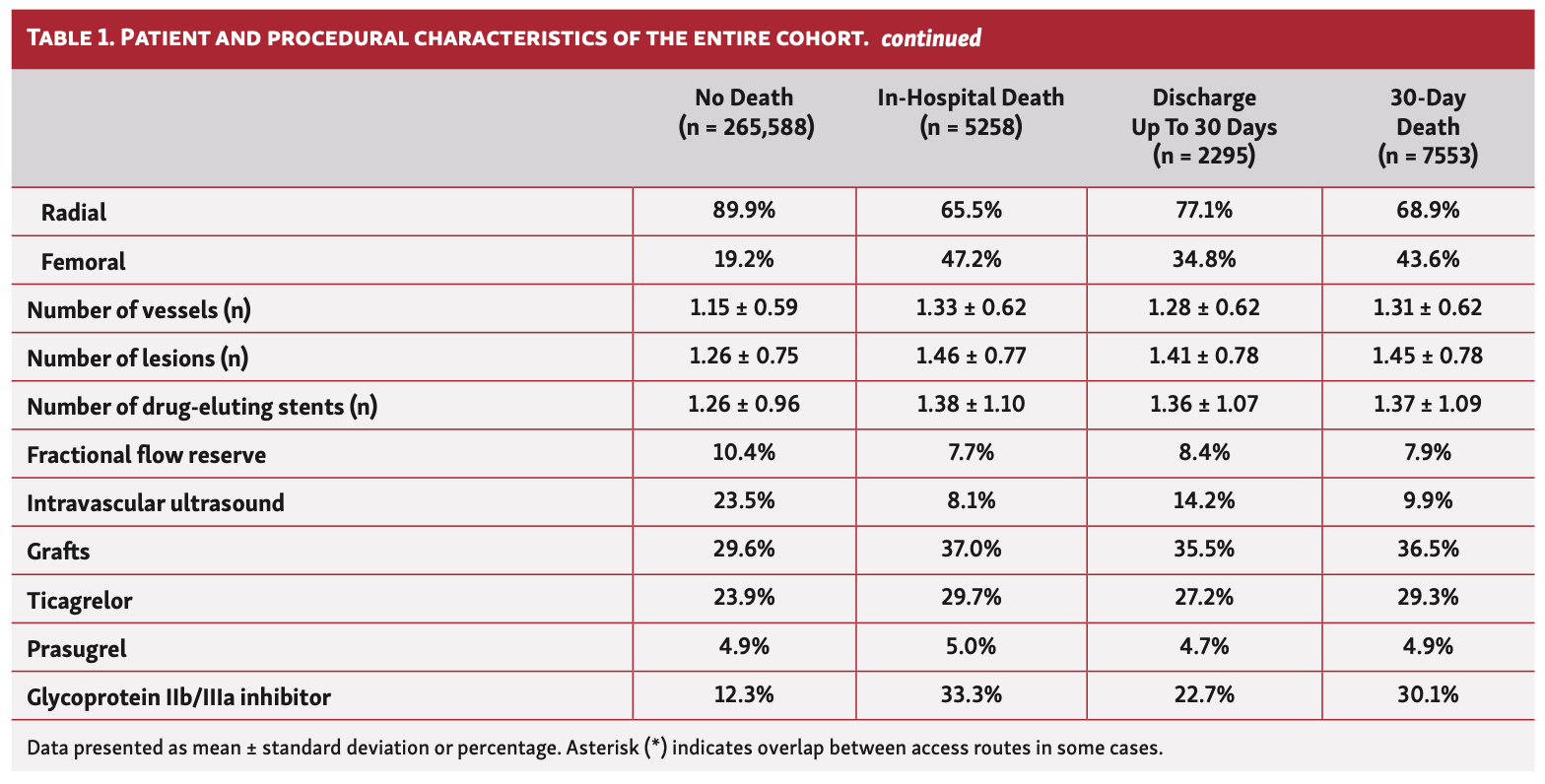
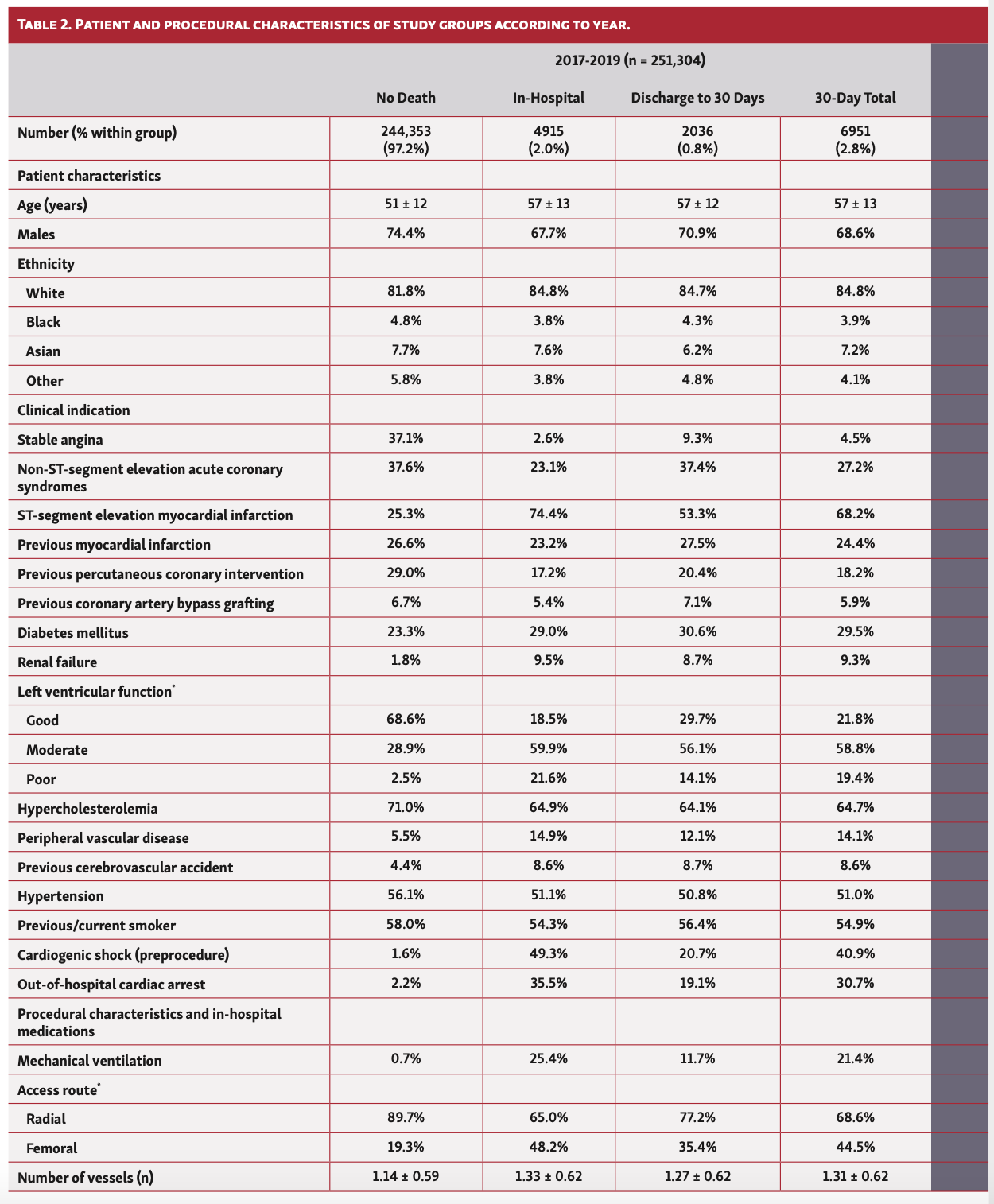
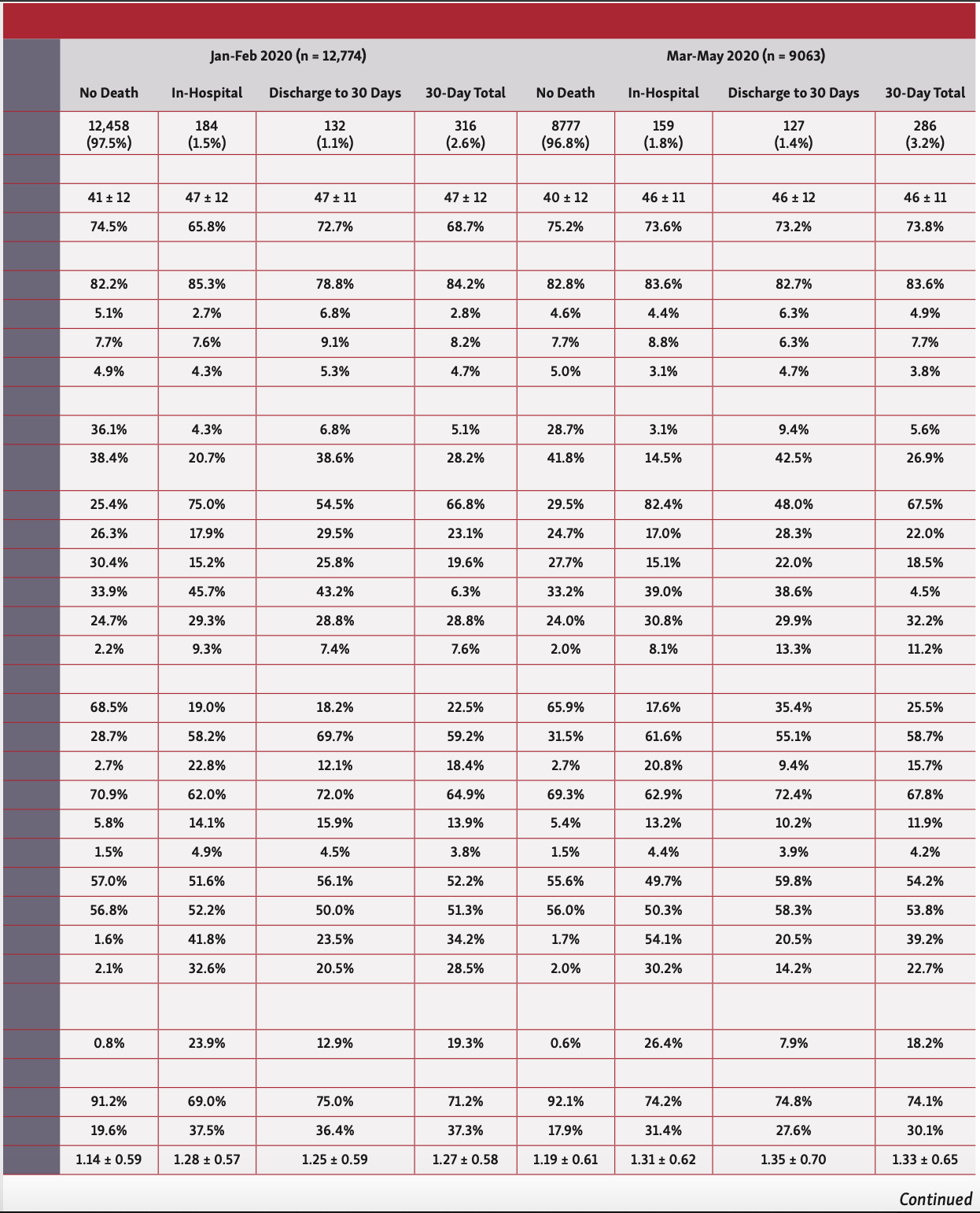
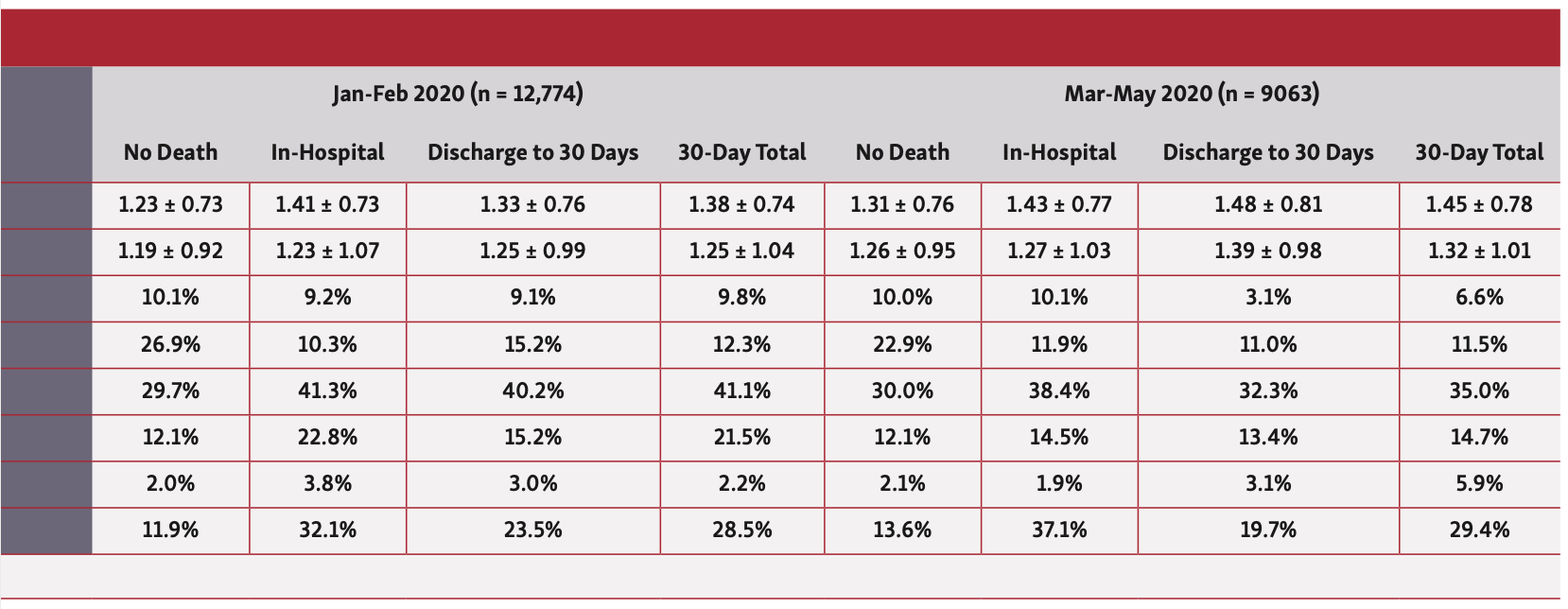
Overall, patients who died in hospital or at 30 days were older (57 years vs 51 years), less likely to be males or from ethnic minorities, and more likely to have an ACS indication for their PCI. They had a greater prevalence of cardiovascular risk factors including previous coronary artery bypass grafting (CABG), diabetes, renal failure, moderate-poor left ventricular (LV) function, peripheral vascular disease (PVD), and previous cerebrovascular accidents (CVAs) (Table 1). Furthermore, patients who died in hospital and at 30 days were significantly more likely to have been in cardiogenic shock or suffered cardiac arrest or received mechanical ventilation prior to PCI. While this pattern of findings was consistent across the study years, patients undergoing PCI in 2020, particularly between March and May, were significantly younger (41-47 years vs 51-57 years) (Table 2).
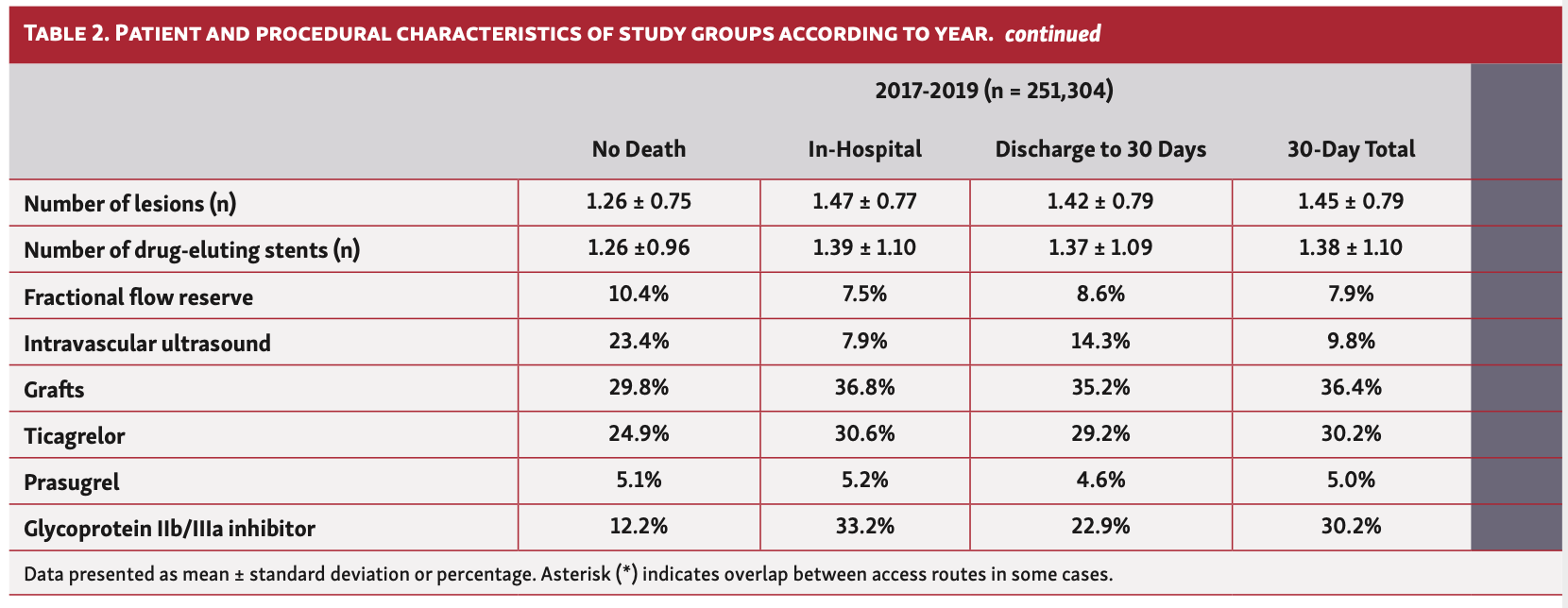
In terms of procedural characteristics, patients who died in hospital or at 30 days were more likely to undergo a procedure via femoral access (34.8%-47.2% vs 19.2%), for grafts (35.5%-37.0% vs 29.6%) and to have multivessel disease (mean number of vessels, 1.28-1.33 vs 1.15) as well as multiple lesions (mean number of lesions, 1.41-1.46 vs 1.25), using a greater number of drug-eluting stents (mean number of stents, 1.36-1.38 vs. 1.26) compared with those who did not die (Table 1). Furthermore, they were less likely to undergo intravascular ultrasound (IVUS) imaging (8.1%-14.2% vs 23.5%) and fractional flow reserve (FFR) assessment (FFR, 7.7%-8.4% vs 10.4%), and more likely to receive glycoprotein IIb/IIIa inhibitors (22.7%-33.3% vs 12.3%). Patients who died within 30 days were also more likely to receive ticagrelor (27.2%-29.7% vs 23.9%), but no difference in the utilization of prasugrel was observed between groups. These findings persisted throughout the study period (Table 2).
30-Day Causes of Death
One in 10 deaths (10.4%) at 30 days were due to confirmed COVID in those who underwent PCI between March 1, 2020 and May 10, 2020 (Table 3A). Overall, the majority of deaths within 30 days were cardiovascular in origin (59.6%; n = 4499), and this persisted between 2017 and 2020 (Figure 4). Cardiac deaths were mostly due to ACS (33.2%) and cardiogenic shock (10.2%), moreso in in-hospital deaths than postdischarge deaths, followed by heart failure (7.5%), which was higher in postdischarge deaths than in-hospital deaths (30-day death illustrated in Figure 5). While this pattern was consistent over the study period, the rate of in-hospital death due to cardiogenic shock significantly increased between March 1, 2020 and May 10, 2020 (17.6% vs 9.4%-12.8%) (Table 3B). The most common causes of non-cardiac death were hypoxic brain injury for in-hospital death (10.9%) and non-COVID infections for postdischarge death (4.2%) (Table 3A). Deaths due to infections (non-COVID) increased in 2020, both in hospital and post discharge, whereas deaths due to hypoxic brain injury declined over the same period, particularly post discharge (Table 3B).
When stratified by PCI indication, the majority of 30-day deaths occurred in patients who underwent PCI for ACS (n = 7205; 95.4% of all deaths). Within this group, the most common cause of 30-day death was cardiac in origin (60.2%), with a third of deaths primarily due to ACS (33.9%). Hypoxic brain injury and cardiogenic shock were the most prevalent other causes (11.3% and 10.6%, respectively), all of which were more likely to contribute to in-hospital than postdischarge death (Supplemental Table S3). In patients who underwent PCI for stable angina, the most common causes of 30-day death were non-cardiac (53.6%). The most common cardiac causes of 30-day death in the stable angina group were ACS (17.2%), heart failure (14.1%), and pre-existing heart disease (8.6%).
Predictors of Death
Several factors correlated with increased odds of both 30-day cardiac and non-cardiac, including advanced age (>50 years), ACS compared with stable angina (ST-segment elevation myocardial infarction [STEMI] > non-ST-segment elevation acute coronary syndrome [NSTEACS]), adverse events prior to PCI including cardiogenic shock, out-of-hospital cardiac arrest, and need for pre-PCI mechanical ventilation, renal failure, moderate-poor LV function, peripheral vascular disease, diabetes mellitus, and history of myocardial infarction or cerebrovascular accident (Table 4). Among the procedural characteristics, the odds of 30-day cardiac death were increased with femoral access (OR, 1.62; 95% CI, 1.47-1.77), graft PCI (OR, 1.24; 95% CI, 1.16-1.32), and greater number of lesions (per additional lesion: OR, 1.15; 95% CI, 1.06-1.23) or vessels (per additional vessel: OR, 1.34; 95% CI, 1.23; 1.46) (P<.001 for all). In contrast, the odds of 30-day cardiac and non-cardiac death were lower among males (OR, 0.70 [95% CI, 0.66-0.75] and OR, 0.77 [95% CI, 0.72-0.83], respectively; P<.001 for both) and non-white ethnic backgrounds (Table 4). Furthermore, the odds of 30-day cardiac death were reduced with radial access (OR, 0.78; 95% CI, 0.70-0.86) and the use of IVUS (OR, 0.75; 95% CI, 0.67-0.83), FFR (OR, 0.81; 95% CI, 0.72-0.91), and drug-eluting stent (OR, 0.78; 95% CI, 0.68-0.89) (P<.001 for all). The odds of COVID-related 30-day death were significantly increased in patients ages 60-69 years (OR, 5.61; 95% CI, 2.07-15.16; P<.01) and those with renal failure (OR, 7.31; 95% CI, 2.66-20.06; P<.001). Although there was a trend toward increased COVID-related death among Black and Asian ethnicities (OR, 2.95 [95% CI, 0.98-8.84] and OR, 1.70 [95% CI, 0.55-5.26], respectively) and males (OR, 1.59; 95% CI, 0.60-4.23), this was not statistically significant, possibly due to the relatively small number of COVID-19 deaths.
Discussion
The present study highlights several important findings from a contemporary nationwide cohort of patients treated by PCI in England. First, we found that only 2 of every 3 deaths at 30 days post PCI were from cardiac causes, and that this persisted throughout the study period, even during the COVID-19 pandemic. Patients treated for an ACS accounted for a third of 30-day deaths throughout the study, followed by hypoxic brain injury and cardiogenic shock as the most prevalent causes. Second, we observed that in-hospital and 30-day deaths after PCI, from both cardiac and non-cardiac causes, declined in the past 3 years, although these have risen again after the start of the COVID-19 pandemic (March 1, 2020 through May 10, 2020), primarily due to non-cardiac deaths. One in 10 deaths at 30 days were due to COVID-19 in patients undergoing PCI between March and May 2020.
Some previous studies have suggested that short-term mortality (≤30 days) after PCI is predominantly due to cardiac causes, while others have shown otherwise.6,7,17 However, there are limited data on the causes of death after PCI in the current era, and how these have changed from a national perspective. Previous studies provided conflicting information, likely due to variations in the cohorts they had examined, which included individual centers15 or healthcare systems,16 or only inpatient procedures, with no analysis of postdischarge causes of death.5 Pooled data from 21 RCTs demonstrated a 7-fold higher rate of CV than non-CV deaths in 32,882 patients undergoing PCI (relative ratio, 6.99; 95% CI, 3.16-15.42).7 Despite the added value of this study, patients enrolled in RCTs are highly selected cohorts that are often healthier than the background population encountered in daily practice. Furthermore, the authors mention that death of unknown cause in RCTs was adjudicated as CV in origin as per protocol, which may have influenced the external validity of their findings. In contrast, a study by Bricker et al reported higher rates (~60%) of non-CV/undifferentiated deaths at 30 days in 115,191 patients undergoing PCI in the VA healthcare system in the United States. However, their study examined a selected cohort of veterans, who were predominantly males (99%) and generally older than the average population.16 Furthermore, deaths that occurred outside the VA were not captured, which may explain their apparently high number of non-cardiac deaths.
Although the majority of in-hospital and 30-day deaths in our cohort were cardiac in origin, 4 out of 10 deaths (~40%) were non-cardiac, with hypoxic brain injury and infections being the most common non-cardiac causes, a finding that persisted over the study period. This finding raises questions regarding the utility of currently used PCI mortality risk scores, such as the New York State Risk (NYSR) score and National Cardiovascular Data Registry (NCDR) score.6,8,27 While these scores have been validated for the prediction of overall in-hospital mortality, they do not discriminate between CV and non-CV deaths, with the latter representing a significant proportion of all in-hospital deaths. Furthermore, these risk scores were derived from relatively outdated cohorts (eg, NCDR: 2004 to 2006; NYSR: 2009 to 2010), of which procedural characteristics are different from the current era. Similarly, an argument could be made that public reporting of operator outcomes should detail the broad nature of the cause of death, particularly in view of the cause of death distribution during the COVID-19 pandemic, where 1 in 10 deaths at 30 days were due to COVID-19. Interestingly, most of the COVID-19 deaths in our cohort were among patients undergoing PCI for stable angina, although it does not determine whether the transmission of COVID-19 was nosocomial or post discharge in the community. Nevertheless, this raises a concern regarding the safety of performing non-emergent PCI until the pandemic has subsided.
There are limited data regarding trends in in-hospital and 30-day mortality in recent years. In a single-center study of 19,506 patients undergoing PCI at Mayo Clinic in Rochester, Minnesota, Spoon et al reported a decline for in-hospital death rates between 1991 (2.7%) and 2006 (2.2%), as well as a reduction in long-term (5-year) cardiac death (33% temporal decline), with a parallel increase in long-term non-cardiac death (57%).15 Their findings, however, were derived from a relatively old cohort that was less likely to receive drug-eluting stent implantation and newer P2Y12 inhibitors, and one that was managed in a large tertiary facility and, thereby, does not reflect contemporary national-level practices or outcomes. Furthermore, their analysis does not provide information on the cause of in-hospital mortality (eg, cardiac vs non-cardiac), and only considered this aspect for long-term outcomes. In contrast, Alkhouli et al reported an increase in in-hospital mortality after PCI between 2003 and 2016, both in ACS and stable angina patients, in a 20% stratified national sample of United States hospitalizations.5 While their analysis provides us with insights into the trends of in-hospital mortality in inpatients undergoing PCI, it does not inform us on outcomes for outpatient procedures, which represent a large proportion of all PCI cases. Moreover, they did not report the cause of in-hospital mortality. Our findings suggest that both 30-day cardiac and non-cardiac death rates after PCI have declined over the past 3 years, mainly driven by a fall in in-hospital death, up until the start of the COVID-19 pandemic (March 1, 2020), after which there was an increase in both in-hospital cardiac and non-cardiac deaths. The latter could be explained by baseline differences between patients undergoing PCI before and after the pandemic. For example, patients undergoing PCI during the pandemic could have been more critically unwell, or higher-risk PCI cases, although we note that patient and procedural characteristics during both time periods were relatively similar. Another possible explanation is the delayed presentation of ACS cases during the pandemic, which have been widely reported in recent literature, as well as less-optimal care for critically ill cases due to pressures on hospital systems as a result of the COVID-19 pandemic.28-30
Strengths and limitations. The present study is the largest to report trends of 30-day causes of deaths after PCI in a contemporary nationwide cohort, including both inpatient and outpatient procedures, making it generalizable to the wider population of interest. Another notable strength is our ascertainment and determination of cause of death, which is based on linkage to the ONS database and details from the Medical Cause of Death Certificate, unlike some previous studies. However, there are several limitations to the present study. First, while the BCIS dataset captures CV risk factors and procedural characteristics, it does not capture measures of comorbidity, such as Charlson or Elixhauser scores, or frailty that are important determinants of mortality post PCI.31,32 The observational nature of our study means that residual unmeasured confounders such as these and others may not be accounted for. Third, given that these data are the most contemporary available (census June 2020 for procedures in May 2020) there is insufficient follow-up to study longer-term causes of death, particularly given that it has been shown in patients derived from RCTs that non-cardiovascular causes of mortality become more important at longer-term follow-up.7 Finally, COVID-19 cases were identified according to the corresponding ICD-10 code (U07.1 – confirmed COVID). However, the data do not inform us of whether this confirmation was based on virology/serology or clinical diagnoses.
Conclusions
In a nationwide cohort of PCI procedures, we demonstrate that a significant proportion of 30-day deaths is due to non-cardiac causes, a finding that has persisted over the past 3 years. Overall, in-hospital and 30-day cardiac and non-cardiac deaths have declined over 3 years, before increasing again during the COVID-19 pandemic. One in 10 deaths in those undergoing PCI from March 1, 2020 were due to COVID-19. The present findings highlight that overall 30-day mortality may not be a reliable measure of performance in the context of PCI, and drive the need for other objective measures, which may include cause-specific mortality.
Acknowledgments. The authors acknowledge Chris Roebuck and Tom Denwood from NHS digital for providing and creating the secure environment for data hosting and for analytical support.
From the 1Keele Cardiovascular Research Group, Centre for Prognosis Research, Keele University, United Kingdom; 2Department of Cardiology, Royal Stoke University Hospital, Stoke-on-Trent, United Kingdom; 3Department of Cardiology, University Hospital of Wales, Cardiff, Wales, United Kingdom; 4Wessex Cardiothoracic Unit, Southampton University Hospital Southampton & Faculty of Medicine University of Southampton, United Kingdom; 5Institute of Cardiovascular Sciences, University of Birmingham, Birmingham, United Kingdom; 6Leeds Institute for Data Analytics, University of Leeds, Leeds, United Kingdom; 7National Institute for Cardiovascular Outcomes Research, Barts Health NHS Trust, London, United Kingdom; 8Institute of Cardiovascular Sciences, University College London, London, United Kingdom; 9Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, United Kingdom; and 10Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript accepted December 4, 2020.
Address for correspondence: Mamas A. Mamas, DPhil, Professor of Cardiology, Keele Cardiovascular Research Group, Centre for Prognosis Research, Institute for Primary Care and Health Sciences, Keele University, UK. Email: mamasmamas1@yahoo.co.uk
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
- Weiss AJ, Elixhauser A. Trends in operating room procedures in U.S. hospitals, 2001-2011: statistical brief #171. Healthcare cost and utilization project (HCUP) statistical briefs. Rockville (MD): Agency for Healthcare Research and Quality (U.S.). 2006.
- Qian F, Zhong Y, Hannan EL. Relation between operator and hospital volumes and long-term outcomes for percutaneous coronary intervention in New York. Am J Cardiol. 2020;125:694-711.
- Gajanana D, Weintraub WS, Kolm P, et al. Trends in death rate 2009 to 2018 following percutaneous coronary intervention stratified by acuteness of presentation. Am J Cardiol. 2019;124:1349-1356.
- Alkhouli M, Alqahtani F, Kalra A, et al. Trends in characteristics and outcomes of hospital inpatients undergoing coronary revascularization in the United States, 2003-2016. JAMA Network Open. 2020;3:e1921326-e1921326.
- Peterson ED, Dai D, DeLong ER, et al. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55:1923-1932.
- Brener SJ, Tarantini G, Leon MB, et al. Cardiovascular and noncardiovascular death after percutaneous coronary intervention. Circ Cardiovasc Interv. 2018;11:e006488.
- Hannan EL, Farrell LS, Walford G, et al. The New York State risk score for predicting in-hospital/30-day mortality following percutaneous coronary intervention. JACC Cardiovasc Interv. 2013;6:614-622.
- Dunt D, Prang KH, Sabanovic H, Kelaher M. The impact of public performance reporting on market share, mortality, and patient mix outcomes associated with coronary artery bypass grafts and percutaneous coronary interventions (2000-2016): a systematic review and meta-analysis. Med Care. 2018;56:956-966.
- Jones DA, Rathod KS, Koganti S, et al. The association between the public reporting of individual operator outcomes with patient profiles, procedural management, and mortality after percutaneous coronary intervention: an observational study from the Pan-London PCI (BCIS) registry using an interrupted time series analysis. Eur Heart J. 2019;40:2620-2629.
- Wadhera RK, Bhatt DL. Taking the "public" out of public reporting of percutaneous coronary intervention. JAMA. 2017;318:1439-1440.
- Wadhera RK, Bhatt DL. Toward precision policy — the case of cardiovascular care. N Engl J Med. 2018;379:2193-2195.
- Wadhera RK, Joynt Maddox KE, Yeh RW, Bhatt DL. Public reporting of percutaneous coronary intervention outcomes: moving beyond the status quo. JAMA Cardiol. 2018;3:635-640.
- Wang DE, Wadhera RK, Bhatt DL. Public reporting of percutaneous coronary interventions. Med J Aust. 2018;209:104-105.
- Spoon DB, Psaltis PJ, Singh M, et al. Trends in cause of death after percutaneous coronary intervention. Circulation. 2014;129:1286-1294.
- Bricker RS, Valle JA, Plomondon ME, Armstrong EJ, Waldo SW. Causes of mortality after percutaneous coronary intervention. Circ Cardiovasc Qual Outcomes. 2019;12:e005355.
- Aggarwal B, Ellis SG, Lincoff AM, et al. Cause of death within 30 days of percutaneous coronary intervention in an era of mandatory outcome reporting. J Am Coll Cardiol. 2013;62:409-415.
- 2020. Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU).
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069.
- Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. COVID-19 and the cardiovascular system. Nature Reviews Cardiol. 2020;17:259-260.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062.
- Clerkin KJ, Fried JA, Raikhelkar J, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648-1655.
- Ludman PF. British Cardiovascular Intervention Society registry for audit and quality assessment of percutaneous coronary interventions in the United Kingdom. Heart. 2011;97:1293-1297.
- Mamas MA, Nolan J, de Belder MA, et al. Changes in arterial access site and association with mortality in the United Kingdom: observations from a national percutaneous coronary intervention database. Circulation 2016;133:1655-1667.
- (ONS) OfNS. Coronavirus (COVID-19) roundup; 2020.
- Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley & Sons, Inc., New York: 1987.
- McAllister KSL, Ludman PF, Hulme W, et al; for the British Cardiovascular Intervention Society. A contemporary risk model for predicting 30-day mortality following percutaneous coronary intervention in England and Wales. Int J Cardiol. 2016;210:125-132.
- Cosentino N, Assanelli E, Merlino L, Mazza M, Bartorelli AL, Marenzi G. An in-hospital pathway for acute coronary syndrome patients during the COVID-19 outbreak: initial experience under real-world suboptimal conditions. Can J Cardiol. 2020;36:961-964.
- Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871-2872.
- Toner L, Koshy AN, Hamilton GW, Clark D, Farouque O, Yudi MB. Acute coronary syndromes undergoing percutaneous coronary intervention in the COVID-19 era: comparable case volumes but delayed symptom onset to hospital presentation. Eur Heart J Qual Care Clin Outcomes. 2020;6:225-226.
- Mamas MA, Fath-Ordoubadi F, Danzi GB, et al. Prevalence and impact of co-morbidity burden as defined by the Charlson co-morbidity index on 30-day and 1- and 5-year outcomes after coronary stent implantation (from the Nobori-2 study). Am J Cardiol. 2015;116:364-371.
- Potts J, Nagaraja V, Al Suwaidi J, et al. The influence of Elixhauser comorbidity index on percutaneous coronary intervention outcomes. Catheter Cardiovasc Interv. 2019;94:195-203.