Feasibility and Safety of the Second-Generation, Frequency Domain Optical Coherence Tomography (FD-OCT): A Multicenter Study
Abstract: Objectives. This study sought to assess the effectiveness and safety of the second-generation frequency-domain optical coherence tomography (FD-OCT) system. Background. The second-generation FD-OCT was recently developed, with simplified imaging technique and faster acquisition time compared to the first-generation time-domain OCT. However, the safety and effectiveness of the FD-OCT has not been evaluated, and this study was conceived as a pre-approval study for Food and Drug Administration clearance for clinical use in the United States. Methods. A total of 50 patients were enrolled from 3 institutions. Following stent implantation, the FD-OCT was performed with contrast injection through the guiding catheter to acquire pullback images with the pressure-triggered automatic pullback device. The primary endpoint was to achieve a median clear image length of more than 24 mm. Serious procedure-related or postprocedural adverse events (death, myocardial infarction, or ventricular arrhythmia) were recorded to assess safety of the device. Results. The primary endpoint of obtaining >24 mm of median clear image length (CIL) was achieved in 94% of the subjects (47 out of 50), with measured CIL of 43.2 mm. In 5 patients (10.6 %), a second attempt was necessary due to suboptimal image quality of the first pullback. In 36 patients (76.6%), a full stent length was obtained during the first attempt. There were no serious procedure-related or postprocedural adverse events. Conclusions. The new second-generation FD-OCT system provides fast and reliable resolution images of the coronary artery. The pullback can be safely performed over long segments of the artery without serious adverse events.
J INVASIVE CARDIOL 2012;24(5):206-209
Key words: frequency domain OCT, FD-OCT, feasibility, safety
______________________________________________
Intracoronary optical coherence tomography (OCT) is a high-resolution imaging modality that uses near-infrared light to visualize coronary artery structures and geometry. Since its first use for in vivo coronary artery evaluation in 2002,1 it has been widely accepted as a reliable tool for assessing the structures of coronary arteries. With OCT, it is possible to visualize pathologic changes in microstructures of the coronary artery, as well as evaluate the adequacy of stent deployment due to its higher resolution (10 µm) compared to intravascular ultrasound (IVUS; resolution, 100 µm).1
One limitation of OCT compared to IVUS is the requirement for blood to be displaced in order to obtain images of the artery wall. The techniques for blood displacement in the conventional time-domain OCT (TD-OCT) require either the combined use of a small imaging catheter and a compliant occlusion balloon (occlusive technique) or an infusion of an iso-osmolar viscous solution through the guiding catheter (non-occlusive technique).2 These techniques have been proven to be safe, but require extra time to perform and may not be feasible in clinically unstable patients.3,4 Additionally, the TD-OCT has a slow pullback rate of about 0.5-3 mm/s, with 10-30 seconds of occlusion or flush to image a 30-mm long coronary artery, which may lead to myocardial ischemia.4
The second-generation frequency-domain OCT (FD-OCT) system has recently been developed for clinical use. It employs a rapid-exchange design non-occlusive catheter, which minimizes the possible ischemic effect caused by occlusion of coronary arteries. The FD-OCT system provides faster acquisition time with a pullback speed of 20 mm/s that can scan up to 50 mm of an artery in about 3 seconds.5 Additionally, there is increased penetration depth6 and a higher sampling line density per frame with the FD-OCT5 compared to TD-OCT. This multicenter study was performed as a pre-approval study for Food and Drug Administration (FDA) clearance, in order to evaluate the effectiveness and safety of the newly developed FD-OCT for imaging intracoronary stents.
Methods
Study population. The multicenter study included 3 medical centers and was conducted from November 2008 to July 2009. The inclusion criteria were adult (age >18 years) patients with stable angina or acute coronary syndrome (ACS) who were undergoing percutaneous coronary intervention (PCI) with a single stent.
Clinical exclusion criteria were serious co-morbid conditions (malignancy, inflammatory disease), previous coronary artery bypass graft surgery in the target vessel, New York Heart Association class III or IV heart failure, left ventricular ejection fraction <30%, ACS within 24 hours before the index procedure, hemodynamic or electrical instability, and renal insufficiency (serum creatinine ≥1.8 mg/dL). The lesion-specific exclusion criteria were lesions with multiple stents, stent diameters <2.0 mm or >3.5 mm, ostial lesions, lesions containing large thrombus, and heavily calcified or highly tortuous vessels. The study protocol was approved by the institutional review board and ethics committee at each participating institution, and written informed consent was obtained from all participating patients.
Image acquisition. Femoral approach was used in all patients for coronary angiograms, PCI and OCT imaging. Weight-adjusted, unfractionated heparin (institution M and institution S) or bivalirudin (institution C) was given for anticoagulation. After the placement of the guiding catheter (6 Fr) into the coronary ostium, a standard PCI guidewire was advanced into the coronary artery in the conventional manner. Following the placement of an FDA-approved intracoronary stent, FD-OCT imaging was performed.
FD-OCT system and catheter. The FD-OCT system (C7 System; LightLab Imaging, Inc/St Jude Medical) consists of a computer console containing the data acquisition board, an imaging engine, and a patient interface unit that connects to the intravascular OCT catheter (RX Image wire II; LightLab Imaging, Inc/St Jude Medical), In this study, the 2.7 Fr rapid-exchange catheter was advanced over a 0.014˝ coronary guidewire through a 6 Fr guide catheter. The imaging lens of the catheter was positioned distal to the stent. After catheter placement, a cardiovascular injection pump was used to deliver contrast medium through the guide catheter at a rate of 4 mL/s. An average of 14 cc of contrast medium was used for each run. The optical fiber of the catheter was automatically pulled back at a rate of 20 mm/s for 2.5 seconds.
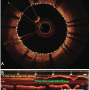 Image analysis. Images were digitally stored and submitted to the core laboratory for offline analysis. OCT images were analyzed by two independent observers who were blinded to the procedure and patient information. Each OCT pullback was analyzed every 1 mm to measure the overall length of the pullback containing clear cross-sectional images.
Image analysis. Images were digitally stored and submitted to the core laboratory for offline analysis. OCT images were analyzed by two independent observers who were blinded to the procedure and patient information. Each OCT pullback was analyzed every 1 mm to measure the overall length of the pullback containing clear cross-sectional images.
A recent analysis of a large stent implantation database has demonstrated that 90% of implanted coronary stents have a length less than 24 mm.7,8 Based on this statistic, our study selected a performance target of 24 mm for the median length of clear imaging within an OCT pullback. The clear imaging length (CIL, in millimeters) is the cumulative sum of clear image segment frames (CIF). The CIF was defined as an OCT cross-sectional image frame with a visible boundary between the lumen and the vessel wall along a continuous arc of at least 270° around the center of the lumen (Figure 1A). In addition, length of stent that was visible within the CIL was identified. Clear stent length (CSL) was defined as the total stent length (in millimeters) visualized within the CIL (Figure 1B).
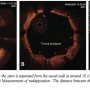 Additional features including presence of stent edge dissection, malapposition, and tissue prolapse were recorded during analysis (Figure 2). Stent edge dissection was defined as a linear rim of tissue clearly separated from the vessel wall or the adjacent stent struts at the stent edge.9Malapposition was noted when the distance from the center of the superficial reflection in stent strut to the leading edge of the vessel wall was greater than the sum of strut thickness plus polymer.9Tissue prolapse was defined as the presence of tissue between stent struts protruding into the lumen with a circular arc connecting adjacent struts.
Additional features including presence of stent edge dissection, malapposition, and tissue prolapse were recorded during analysis (Figure 2). Stent edge dissection was defined as a linear rim of tissue clearly separated from the vessel wall or the adjacent stent struts at the stent edge.9Malapposition was noted when the distance from the center of the superficial reflection in stent strut to the leading edge of the vessel wall was greater than the sum of strut thickness plus polymer.9Tissue prolapse was defined as the presence of tissue between stent struts protruding into the lumen with a circular arc connecting adjacent struts.
Primary endpoint. The primary endpoint was to achieve a median clear image length of more than 24 mm to demonstrate diagnostic effectiveness. The safety endpoint was assessed by the incidence of serious procedural or postprocedural adverse events, including angina pectoris, ST-segment changes, or arterial spasm persisting despite treatment, ventricular arrhythmias, coronary artery dissection, intracoronary thrombus, periprocedural myocardial infarction, or death.
Secondary endpoints. The CSL was evaluated to examine the completeness of stent length that could be visualized during imaging. Changes in intracoronary microstructures including edge dissection, tissue prolapse, and malapposition were documented to represent the additional diagnostic feasibility.
Intraobserver and interobserver variabilities. CIL was evaluated by two independent observers to assess interobserver variability. CIL measurement was repeated by each observer at least 4 weeks after the initial evaluation to assess intraobserver variablity. When there was discordance between observers, a consensus reading was obtained.
Statistical analysis. Continuous variables were reported as the mean and standard deviation (SD) or the median and interquartile range based on their normal distribution. Categorical variables were expressed as the number or frequency of occurrence.
Results
 The baseline characteristics are shown in Table 1. The majority of patients had stable angina with preserved left ventricular function. A total of 52 OCT image pullbacks from 47 patients were performed. The total number of frames evaluated in this study was 10,195 (2039 mm), and the total stent length analyzed was 733.3 mm (7388 struts). The target vessel was the left anterior descending coronary artery in 20 patients, the left circumflex artery in 8 patients and the right coronary artery in 19 patients.
The baseline characteristics are shown in Table 1. The majority of patients had stable angina with preserved left ventricular function. A total of 52 OCT image pullbacks from 47 patients were performed. The total number of frames evaluated in this study was 10,195 (2039 mm), and the total stent length analyzed was 733.3 mm (7388 struts). The target vessel was the left anterior descending coronary artery in 20 patients, the left circumflex artery in 8 patients and the right coronary artery in 19 patients.
Effectiveness assessment. The primary endpoint of obtaining greater than 24 mm of CIL was achieved in 94% of the subjects (47 out of 50). Technical failures occurred in 3 cases: failure to obtain images in 2 cases secondary to the guidewire malfunctioning, and console error secondary to software failure in 1 case. In 5 of 47 patients (10.6%), a second attempt was necessary due to the suboptimal image quality of the first pullback. In all 5 patients, the second attempt was necessary due to residual blood artifacts. Though full-length CIL was obtained in the second attempt, the CSL was not measurable in 1 of the 5 patients. There was no significant artifact such as sew-up artifact (a cut-off line in image created by the movement of the heart during the OCT pullback) that led to the second attempt of OCT. In the remaining 42 patients (89.4%), a clear image was obtained during the first attempt. From all 47 patients, the measured median length of CIL was 43.2 mm. The interobserver and intraobserver correlation coefficients for CIL were 0.96 and 0.98, respectively.
Safety assessment. Transient chest discomfort and bradycardia were observed in 5 patients (10.6%) and 1 patient (2.1%), respectively, during the OCT procedure. All events were transient and were resolved without complication. No procedural complications were observed, including acute vessel occlusions, angiographically noticeable dissections, thrombi formation, emboli, or vasospasms. There were no serious postprocedural adverse events noted, including postprocedure myocardial infarction, urgent revascularization, or death.
Stent characteristics. Out of the 47 patients, 46 included stents that were measurable for CSL. The average measured CSL was 15.9 ± 4.4 mm. From the procedural information, the average stent size of the study group was calculated to be 16.7 ± 3.7 mm. We found 502 struts (6.8%) to be malapposed from a total of 7388 struts. Edge dissection and tissue prolapse were detected in 10.6% and 83.0% of stents, respectively.
Discussion
This multicenter study was performed to evaluate the feasibility and safety of the newly developed FD-OCT in patients undergoing elective PCI. In a single-center report, the conventional TD-OCT was able to successfully image 80.8% of target segments,5 with the approximately 20% failure rate mostly due to non-life-threatening complications. In this study, the procedure success rate of CIL >24 mm was 94.0% with no procedure-related complications. This suggests that the new FD-OCT appears to be superior to TD-OCT in acquiring successful images, which may be mostly driven by the lower incidence of artifacts. For example, Takarada et al5 reported sew-up artifacts in 16.9% of segments (88 segments out of 520) from TD-OCT images, which led to multiple attempts in each patient to obtain a clear image. In our study, none of the sew-up artifacts interrupted plaque or stent analysis. This reflects the superiority of the higher pullback speed (20 mm/s in FD-OCT vs 0.5-3 mm/s in TD-OCT). However, despite the superior image quality with less procedural time, FD-OCT still has only mild improvement in penetrating depth compared to the first-generation OCT. Due to the technical limitation, acquiring optimal image from large vessels (>3.5 mm in diameter) was a challenge.
Out of the 46 cases where stent length was measured, the average CSL was calculated to be 15.9 ± 4.4 mm. From the procedural information, the average stent size in the study was calculated to be 16.7 ± 3.7 mm. The clear stent capture rate (CSL from FD-OCT/actual stent size) in the study was 95.4%, indicating the FD-OCT detected almost all stented segments without an intravascular artifact or a technical difficulty.
 Regarding safety, there were no serious procedure-related adverse events. Transient chest discomfort (5 patients, 10.6%) and bradycardia (1 patient, 2.1%) were observed during the OCT procedure, without persistent ST-segment changes. However, all events were transient and resolved without complication, and none of the events were related to serious adverse outcomes. Transient ischemic electrocardiographic changes were detected in 35% of patients and ventricular ectopic beats were found in 10% of patients with the use of TD-OCT.4 It is suspected that these changes were driven by a longer procedural time and the use of balloon occlusion (Table 2). Although head-to-head comparison was not done, FD-OCT appears to be safer than TD-OCT.
Regarding safety, there were no serious procedure-related adverse events. Transient chest discomfort (5 patients, 10.6%) and bradycardia (1 patient, 2.1%) were observed during the OCT procedure, without persistent ST-segment changes. However, all events were transient and resolved without complication, and none of the events were related to serious adverse outcomes. Transient ischemic electrocardiographic changes were detected in 35% of patients and ventricular ectopic beats were found in 10% of patients with the use of TD-OCT.4 It is suspected that these changes were driven by a longer procedural time and the use of balloon occlusion (Table 2). Although head-to-head comparison was not done, FD-OCT appears to be safer than TD-OCT.
Another report by Takarada et al5 showed that procedures using TD-OCT took three times longer than FD-OCT was used. TD-OCT’s prolonged procedure time was due to switching the OCT wire with guidewire and preparing the occlusion balloon catheter. These steps are simplified using FD-OCT since the catheter employs a rapid-exchange (RX) design with a minirail tip without the need to exchange guidewires. Combined with a higher pullback speed, the development of FD-OCT overcame a major limitation of intravascular OCT imaging. Now it is possible to obtain images through long segments in a short period of time, without increased risk of causing myocardial ischemia.
In this study, edge dissection and tissue prolapse were found in 9.8% and 82.4% of patients, respectively. Malapposition was detected from 6.8% of total struts. The long-term clinical consequence of these intracoronary findings is not known, and it warrants a larger prospective OCT clinical trial or registry-based study.
Study limitations. The first limitation is the small number of patients in this study. However, although the number of patients was small, it is clear that FD-OCT provides adequate information on intravascular structures and is safe. Secondly, FD-OCT was not performed concomitantly with either TD-OCT or IVUS, leaving direct comparisons impossible. Thirdly, patients with complicated coronary morphology (specifically, heavily calcified or highly tortuous vessels) were excluded from the study. All cases were done after PCI with stent deployment, and traversing diseased vessels before PCI was technically not feasible in all cases and excluded. However, these exclusions were used to minimize the potential risk to patients. Also, exclusion of large vessels (>3.5 mm in diameter) was another technical limitation of this study, as large diameter could be a challenge for optimal image acquisition, especially when the catheter is eccentrically positioned.
Conclusion
The FD-OCT system is a new intravascular optical imaging modality with high resolution that can be safely used in patients undergoing PCI.
References
- Jang IK, Bouma BE, Kang DH, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39(4):604-609.
- Prati F, Cera M, Ramazzotti V, et al. From bench to bedside: a novel technique of acquiring OCT images. Circ J. 2008;72(5):839-843.
- Barlis P, Gonzalo N, Di Mario C, et al. A multicentre evaluation of the safety of intracoronary optical coherence tomography. Eurointervention. 2009;5(1):90-95.
- Prati F, Cera M, Ramazzotti V, et al. Safety and feasibility of a new non-occlusive technique for facilitated intracoronary optical coherence tomography (OCT) acquisition in various clinical and anatomical scenarios. Eurointervention. 2007;3(3):365-370.
- Takarada S, Imanishi T, Liu Y, et al. Advantage of next generation frequency-domain optical coherence tomography compared with conventional time-domain system in the assessment of coronary lesion. Catheter Cardiovasc Interv. 2010;75(2):202-206.
- Jang IK, Tearney GJ, MacNeill B, et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111(12):1551-1555.
- Cutlip DE, Baim DS, Ho KK, et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001;103(15):1967-1971.
- Escolar E, Mintz GS, Popma J, et al. Meta-analysis of angiographic versus intravascular ultrasound parameters of drug-eluting stent efficacy (from TAXUS IV, V, and VI). Am J Cardiol. 2007;100(4):621-626.
- Prati F, Regar E, Mintz GS, et al; Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31(4):401-415.
______________________________________________
From the 1Cardiology Division, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, 2Columbia University Medical Center and the Cardiovascular Research Foundation, New York, New York, 3Division of Cardiovascular Medicine, Stanford University Medical Center, Stanford, California, 4LightLab Imaging/St. Jude Medical, Westford, Massachusetts, and 5University Hospitals Case Medical Center, Case Western Reserve University, Cleveland, Ohio.
Funding: This study was funded by a Core Laboratories Analysis Grant.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Jang holds a research grant and is a consultant for St Jude Medical. Dr Bezzera is a consultant for St Jude Medical. Dr Zhang is an employee of St Jude Medical. Dr Costa is a consultant for Abbott and St Jude Medical and has received honoraria from Daiichi Sankyo, Cordis, Scitech, Sanofi, and Medtronic.
Manuscript submitted November 17, 2011, provisional acceptance given January 4, 2012, final version accepted January 20, 2012.
Address for correspondence: Professor Ik-Kyung Jang, Harvard Medical School Cardiology, Massachusetts General Hospital, 55 Fruit Street, Gray/Bigelow 800, Boston, MA, 02114. Email: ijang@partners.org











