ADVERTISEMENT
Excimer Laser Coronary Angioplasty (ELCA): Fundamentals, Mechanism of Action, and Clinical Applications
Abstract: Laser coronary angioplasty was developed to modify atherosclerotic plaque and help with the acute and longer-term limitations of balloon angioplasty, namely, intimal dissection and restenosis, respectively. Excimer laser debulks and modifies the tissue with its photochemical, photothermal, and photokinetic properties without causing significant injury. With important refinements and advancements, laser has gained a renewed place in treating complex and resistant coronary lesions after a disappointing start. When used in line with the instructions, laser is an important tool that allows the completion of difficult and complicated cases. It is a useful tool in the catheterization laboratory to treat lesions that are uncrossable or undilatable. Laser is also helpful in cases where a stent was deployed but remains under-expanded, with accumulating evidence of its efficacy in such cases. In addition, laser is increasingly used for chronic total occlusion (CTO) percutaneous coronary intervention (PCI) to facilitate modification of the proximal CTO cap to allow penetration with a wire and completion of the procedure. Laser has been used in certain circumstances by experienced operators with simultaneous contrast rather than saline injection, to increase its effect and allow the successful completion of complex PCIs. This article outlines the scientific background, experimental data, practical procedural techniques, and clinical applications of excimer laser coronary angioplasty in the treatment of coronary artery disease.
Key words: CTO, complex coronary disease, ELCA, laser angioplasty, percutaneous coronary intervention, PCI, resistant coronary lesions, uncrossable lesions, undilatable lesions
Laser coronary angioplasty was developed to modify atherosclerotic plaque and help with the acute and longer-term limitations of balloon angioplasty, namely, intimal dissection and restenosis, respectively,1 and especially in treating lesions considered not ideal for balloon angioplasty.2 Randomized studies, however, were disappointing particularly with regard to restenosis.3,4 Furthermore, the development and widespread uptake of coronary stents in addition to the high costs of the laser systems resulted in limited development in catheter technology and therefore limited uptake. In recent years, however, there has been renewed interest, with improved understanding of the laser-tissue interface, better technique and equipment, and a realization that its use is best suited to specific circumstances.
This article will outline the scientific background, experimental data, practical procedural techniques, and clinical applications of excimer laser coronary angioplasty (ELCA) in the treatment of coronary artery disease.
Historical Aspects
The use of argon laser angioplasty was first described in animals in 1982.5 In humans, it was first performed in the context of salvaging an ischemic limb in 1983.6 These early applications of argon or Nd:YAG laser technology converted laser light to thermal energy in a continuous wave form to achieve tissue vaporization. This laser thermal angioplasty was met with unsatisfactory results as a result of excessive thermal injury and vessel damage. ELCA was developed to avoid this.
In contrast to continuous-wave laser, pulses lasting nanoseconds (ns) of short-wavelength ultraviolet energy are produced. These can precisely ablate a localized area of atherosclerotic plaque without significant thermal injury.7 After initial laboratory testing, the first successful human ELCA was performed at Cedars-Sinai Medical Center in Los Angeles in 1988.8 In the 1990s, there was an improvement in catheter design from early metal-tipped probes to newer fiber-optic catheters with increased procedural success and lower complication rates.9
The feasibility of a laser guidewire for chronic total occlusions (CTOs) was also shown,10 but later comparison with conventional wires did not show any significant clinical or procedural advantages11 and due to limited use, it is no longer available. Current-generation catheters have either a concentric or eccentric array of a high concentration of laser fibers. Furthermore, operator technique has also evolved with improved safety.
Excimer Laser Fundamentals
Laser is an acronym for Light Amplification by Stimulated Emission of Radiation. It refers to the process of creating a highly directional beam of monochromatic (single-wavelength) light with high energy. The term “excimer” is an acronym for excited dimer. Excimer lasers release energy in the ultraviolet (UV) range (10 to 400 nm) in very short pulses. The advantage of this as compared with lasers that emit in the infrared range is that the absorption depth is less (<100 microns), which reduces the risk of collateral tissue damage. The precise wavelength of emission depends on the exact nature of the gas mixture from which the photons are generated.
Experience in the cardiovascular field has involved the xenon chloride (XeCl) 308 nm laser, which became available in 1983 for research and was approved by the United States Food and Drug Administration for its first clinical indications in 1992. The laser beam is formed as a result of high-voltage electrical discharge across a mixture of the xenon gas and a highly diluted (0.1%) hydrogen chloride solution. An excited state molecule of XeCl (the excited dimer) is produced, which subsequently drops to its ground state of XeCl, a weakly covalent molecule, and liberates a photon with a wavelength of 308 nm. The photon can then interact with another excited electron and produce two photons of the same wavelength and phase. Mirrors are used to amplify this process by reflecting the photons, but also permit emission of the photons to result in the formation of the laser beam.
Mechanisms of action. Excimer laser ablates tissue by three mechanisms:
(1) Photochemical (fracture of molecular bonds): the UV light pulse hits the plaque and is highly absorbed, with each photon generated carrying sufficient energy to break molecular bonds. The duration of the laser pulse is 125 billionths of a second (125 ns), ie, the time that UV light pulse hits the tissue/plaque.
(2) Photothermal (tissue vaporization): the molecular bonds are also vibrated during the absorption process, resulting in heat. Intracellular water is vaporized, leading to cell rupture and the creation of a vapor bubble. This lasts 100 millionths of a second (100 µs).
(3) Photokinetic (clearance of byproducts): the rapid expansion and collapse of the vapor bubble further breaks down plaque, but also assists in clearing byproducts of ablation (water, gases, and small particles). The entire process is completed in 400 µs. The vast majority of these particles are minute enough to be cleared by the reticuloendothelial system, minimizing the risk of distal microembolization (Figure 1A).
Laser equipment. Modern ELCA equipment is manufactured by Philips (formerly Spectranetics). The system consists of a laser unit, which generates the laser beam, and a series of catheters of various sizes (0.9 mm, 1.4 mm, 1.7 mm, 2.0 mm) that transmit this energy by fiber optics to the tip of the catheter, delivering the energy to the intended lesions.
The latest version of the Philips laser unit, the CVX-300 system, is a portable unit that is 35 inches (89 cm) high, 49 inches (124 cm) long, and 24 inches (61 cm) wide, and weighs approximately 650 pounds (295 kg) (Figure 1B). It emits laser energy with a catheter output flow range between 30 and 80 mJ/mm2, a repetition rate of 25-80 pulses/s and a pulse width of 125 to 200 ns (nominal, 135 ns). This unit is also the energy source for other ELCA applications, such as pacemaker lead extraction using the laser sheath and peripheral excimer laser angioplasty.
Available laser catheters include conventional over-the wire laser catheters, which are currently used much less frequently, as well as rapid-exchange or monorail catheters. The rapid-exchange version of the coaxial catheter (Vitesse C and Vitesse Cos; Philips) produces a more-axial force transmission and tip control than the earlier over-the-wire systems. Each coaxial catheter (Vitesse C and Vitesse Cos) consists of 65 to 250 individual 61 µm fibers concentrically arranged around the guidewire lumen. A radiopaque marker is located at the distal end of the catheter to aid localization of the laser tip within the coronary vasculature. The guidewire lumen begins at the tip of the catheter and exits the laser catheter 9 cm from the distal tip (Figure 1C). The Vitesse Cos system is an improved version of the Vitesse C. It consists of a redesigned outer marker band, a smaller guidewire lumen, and optimal spacing of fibers, thereby helping to increase the ablative area and providing more trackability. The concentric catheters were limited primarily to the treatment of concentric lesions and are not suitable for treating highly eccentric plaques.
To overcome this limitation, the Vitesse E series (Philips) of eccentric excimer laser catheters was developed. The catheter shaft consists of an eccentric fiber-optic bundle opposite the guidewire lumen, which runs through a tip with an eccentrically placed guidewire lumen. A radiopaque marker with a radiolucent window is situated at the tip of the catheter. The window aids in directing the tip properly. There is a torque knob that enables the catheter to be rotated so that the fiber-optic bundle is in contact with the plaque.
Currently, the majority of catheters used in practice are concentric. The eccentric catheters are recommended for in-stent restenosis and bifurcation lesions due to the ability to direct the laser beam using the torque knob.
The above-mentioned catheters are available for clinical use (Table 1), as are suggested guiding catheters for the various laser catheters and recommended laser catheter sizes for various vessel sizes.
Procedure
The laser machine requires 5 minutes of start-up time after it is turned on. Prior to the introduction of the laser catheter, it should be calibrated. This is done first by using a calibration catheter that is part of the machine, then by calibrating the sterile catheter that has been chosen for clinical use. The calibration is performed by pointing the tip of the catheter toward the energy detector on the CVX 300 unit and activating the laser by pressing on the foot pedal. The laser will calibrate automatically and enter into a standby mode. Appropriately sized guiding catheters (Table 1) should be used. For most cases, it is preferable to use a supportive large-bore guiding catheter with an adequate lumen that is needed to flush saline using the protocol described below. Guide catheters without side holes improve facilitate infusion.
Once the lesion is crossed with a guidewire, the tip of the guidewire is placed as distal as possible in order to help track the laser catheter along the stiffer part of the wire. All laser catheters are compatible with 0.014˝ guidewires.
ELCA procedural success can be enhanced by using the following key techniques known as the Five Ss of Successful Laser Ablation: Selection of patient; Size of the laser catheter; Settings, fluence, and pulse rate; Saline infusion; and Slow advancement.
(1) Selection of patient. Case selection is paramount to achieve a positive outcome, as demonstrated with ELCA, where selective application has led to the revival of its use. As discussed before, ELCA was initially developed as an alternative to balloon angioplasty, but has now evolved and is seen as an adjunct to conventional PCI.
Table 2 lists the current recommended indications and contraindications for the use of ELCA. Balloon-uncrossable, undilatable lesions remain the main indication. More recently, further niche applications such as stent under-expansion have been described,12 and we now use it routinely for this indication.
Similarly, ELCA has been used in CTO-PCI to help soften and then penetrate the proximal cap (personal experience). We have also described a technique that we termed RASER-PCI, where both laser and rotablation were used, particularly when the rotablation wire would not pass to perform rotational atherectomy, but a standard PCI wire was able to pass. In this situation, laser is used to create a channel through which the rotablation wire was introduced, as laser is less effective in heavy calcification.13
(2) Size of catheter. The maximum diameter of a laser catheter should not exceed two-thirds of the diameter of the artery, as highlighted in Table 1. When selecting the appropriate catheter size, it is best to consider the severity of the lesion in addition to vessel size. Given that ELCA is mainly used for uncrossable or undilatable lesions, a 0.9 mm catheter is usually used. Other instances when it is wise to size conservatively include tortuous vessels (for example, operators should not attempt ELCA in >60° bends as the laser will not bend and may lead to perforation), poorly visualized vessels, and calcified vessels. Larger-sized laser catheters can be considered in large vessels with straight segments and in saphenous vein grafts.
Eccentric catheters can be used in cases of highly eccentric plaques, especially when they are situated at the bend of a vessel, in bifurcation lesions, and in cases of in-stent restenosis.
(3) Settings. The laser catheter is advanced so that the tip is in direct contact with the proximal end of the lesion to minimize the blood interface between the laser tip and the lesion. The fluence, defined as the amount of energy (mJ) at the catheter tip per unit (mm²), usually has a range between 30-80 mJ/mm². The repetition rate (frequency) range is between 25-80 Hz (pulses/s). The system calibrates at 45 mJ/mm² at 25 Hz. The first pass can be made at this setting and increased to maximum settings (Table 1) if there is resistance. The manufacturer recommends increasing the fluence first, and then the frequency if the first activation is not successful. Starting at higher energy and repetition rate may lead to complications, such as dissection and perforation. The authors now start at 45/45 and increase to 60/60 then 80/80 if resistance remains (when using a 0.9 mm catheter, which is the most common catheter size).
(4) Saline flush protocol. The purpose of using a saline infusion is to remove contrast and blood from the lasing field. The 308 nm wavelength photon beam is avidly absorbed by blood and contrast media, leading to the production of insoluble gas and rapidly expanding cavitation bubbles. These bubbles generate intense pressure wave pulses, which are in part responsible for complications, such as dissections and perforations. Knowledge of this deleterious interaction led to the development of the saline flushing technique, which has substantially reduced the severity of coronary dissections, and is now a routine part of the procedure.
A 1 L bag of 0.9% normal saline is attached by means of a sterile intravenous line to one of the ports of a triple manifold via a three-way stopcock. Residual contrast is injected back into the contrast bottle. A fresh 20 mL luer-lock control syringe is attached to another port and is used to flush all traces of blood and contrast from the entire system, including the manifold, Y-connector, guiding catheter, and the target coronary artery with normal saline. Just prior to activation of the laser, the assistant operator injects a 10 mL bolus of normal saline through the guiding catheter, and then continues to inject saline at a rate of 1-3 mL/s during the lasing procedure. Lasing is commenced immediately after the 10 mL bolus of saline. The saline injection is terminated at the end of a lasing sequence. The system will allow lasing for a maximum of 5 or 10 seconds at a time, then automatically enters a 10- or 5-second standby mode, depending on the catheter size. The end of the standby period is marked by an audible signal indicating that the operator can continue. The time of activation can be adjusted, as some operators believe that shorter activation time leads to fewer complications. We mostly use the 0.9 mm catheter, which is activated for 10 seconds and has a standby of 5 seconds.
We have described the use of laser with contrast in certain circumstances such as under-expanded stents or heavily calcified lesions, where a rotablation wire would not pass but a normal PCI wire can.13
(5) Slow advancement. Slow advancement results in larger, “cleaner” lumens and less likelihood of coronary dissection. The catheter is advanced at <1 mm/sec for optimal results. Advancing faster than this defeats the intended purpose of modifying/debulking lesions and instead results in less dilation).
In the early ELCA experience, only one pass would generally be performed through the entire lesion. With the improvements in catheter design and the proper use of saline infusion, operators have been able to optimize results by performing additional laser passes if necessary.
The eccentric laser catheters are rarely used nowadays; however, while using them, there are two methods employed to ablate the tissue. The first method involves simply advancing the catheter through the target tissue, withdrawing the catheter, then repositioning the catheter tip using the torque knob and readvancing. The second method, which has not been used as commonly, involves actively torqueing the catheter at the proximal part of the lesion in an attempt to ablate as much tissue as possible. The operator only advances the catheter when tissue has been removed in a 360° pattern. This method may limit the catheter from slipping into the lumen, which sometimes occurs when using the more commonly applied first method. Good short- and long-term results are ensured by adjunctive angioplasty and stent implantation after the lasing procedure.
Laser Safety
Operators must be trained in the use of laser. It should only be performed in a secure environment with measures taken to warn and prevent unauthorized access during lasing. In addition to these measures, all staff and the patient must wear eye protection (CVX-300 safety glasses; Philips) to protect against harm, mainly from the therapeutic (invisible) laser, which can be absorbed by the cornea. The aiming (red) coherent beam of the laser system can also potentially harm if there is exposure for prolonged periods. The required glasses must be labeled with optical density (OD) at laser wavelength (OD = 4 @ 308 nm).
Avoiding and Managing Complications
ELCA is a procedure that requires careful attention to case selection and technical details. Improved procedural technique, as detailed above, has led to a reduction in the major complications that threatened to limit this procedure during the early experience. The incidence of coronary perforations and major flow-limiting dissections has decreased significantly due to improved catheter design and better operator techniques, including the routine use of saline infusion.
Even with these technical improvements and appropriate case selection, it is important to recognize when ELCA should be abandoned. This is the case when there is failure to pass the catheter when other factors such as guide-catheter selection and guidewire position have been optimized and laser energy is maximized. From a practical point of view, we have had success in many balloon-uncrossable lesions even if the laser catheter did not pass through the lesion and was only activated at the proximal part of the lesion, because the balloon will pass after the activation of high energy, and the procedure can then be performed and finished successfully.
This is the concept behind using laser for modifying the proximal cap of CTOs after penetrating the cap has been difficult. The authors have performed cases of laser at the proximal CTO cap without a wire passing through, which became possible after lasing and allowed the successful completion of the CTO-PCI.
The incidences of ELCA-induced major coronary dissections and abrupt vessel closure, once considered the Achilles’ heel of this procedure, have considerably decreased with the advent of the saline infusion technique in 1995.14 Other measures used to prevent dissections include avoiding excessive force or over-sizing the laser catheter and starting from a low energy and increasing that slowly. If a flow-limiting dissection develops, the laser part of the procedure should be discontinued, and the dissection should be treated with balloon angioplasty and stent placement.
Perforation is also a serious complication and has been reported in 0.3%-2.0% of cases.15,16 Perforations are more likely to occur in the following situations: (1) use of a catheter that is equal to or greater than the vessel diameter; (2) use of a concentric catheter on a very eccentric lesion, particularly if on a tight bend; and (3) when applying laser energy in a previously dissected vascular segment. Once a perforation is diagnosed, the laser catheter is removed without altering the guidewire position and the perforation is treated as per standard practice (often with covered stents).
Clinical studies. Most studies on ELCA were performed in the 1990s. The early experience was mainly recorded in registries. In 1994, Litvack et al published the results of the first 3000 patients (3592 lesions) treated with ELCA at 33 different sites.16 These patients were treated with equipment manufactured by Advanced Interventional Systems (AIS). In this prospective registry, procedural success was 84%, increasing to 90% with adjunctive balloon angioplasty. Procedural complications included significant dissection in 13% and perforation in 1% of lesions. Major ischemic complications were defined as in-hospital mortality (0.5%), Q-wave myocardial infarction (2.1%), and in-hospital bypass surgery (3.8%). Coronary dissection (which occurred in 13% of lesions) was associated with major ischemic complications in approximately 15% of patients.
There was no difference in procedural success or in-hospital complications when the results were analyzed according to American College of Cardiology/American Heart Association (ACC/AHA) lesion type (32% were type C lesions). Use of adjunctive balloon angioplasty increased from 71% in the first 2000 patients to 95% in the last 1000 patients.
The other main manufacturer of laser equipment at the time was Spectranetics. In 1997, the results of the NACI (New Approaches to Coronary Intervention) registry were published.17 In this series, a total of 4429 patients were enrolled from 39 sites between 1990 and 1994. Of these, laser therapy was performed in 887 patients (1000 lesions) with either the AIS system (487 cases) or the Spectranetics system (400 cases). Sixty percent of patients had unstable angina, and ACC/AHA type C lesions were treated in 32% of cases. Procedural success (defined as <50% residual lumen) was 84%. In-hospital mortality was 1.2%, myocardial infarction (MI) occurred in 4.5% of cases (Q-wave in 0.7%), 4.5% required coronary artery bypass grafting (CABG; 2.7% as emergency) with a cumulative death/CABG/MI rate of 9.0%. At 1 year, the incidence of death, Q-wave MI, or target-vessel revascularization was 42.3%. In core-laboratory analysis of 839 lesions (752 patients), post-laser perforations occurred in 2.6% and dissection occurred in 23.4% (22.0% grades B, C, or D and 1.4% grades E or F).
Randomized studies comparing ELCA with balloon angioplasty involved only small numbers of patients. The AMRO (Amsterdam-Rotterdam) trial randomized 308 patients with stable angina to either ELCA (with or without adjunctive balloon angioplasty) or balloon angioplasty.3 The procedural success rate was 80% compared with 79% for balloon angioplasty. Six-month rates of the primary endpoint (death, MI, repeat revascularization) were similar (33% for ELCA vs 30% for balloon angioplasty). Restenosis rates were also similar (52% for ELCA vs 41% for balloon angioplasty; P=.13).
The ERBAC (Excimer laser vs Rotational atherectomy vs Balloon Angioplasty Comparison) trial was a single-center study conducted in Germany.18 This trial randomized 685 patients with stable angina and complex lesions (native type B or C lesions) to either ELCA (n = 232), rotational atherectomy (n = 231), or balloon angioplasty (n = 222). Procedural success was highest in patients who underwent rotational atherectomy (89% vs 77% for ELCA vs 80% for balloon angioplasty; P<.01). There was no difference in major in-hospital complications, defined as death, Q-wave MI, or CABG (3.2% for rotational atherectomy vs 4.3% for ELCA vs 3.1% for balloon angioplasty; P=.71). However, at 6 months, target-lesion revascularization was higher in the rotational atherectomy group (42.4%) and the ELCA group (46.0%) than in the balloon angioplasty group (31.9%; P=.01).
Overall, these early trials demonstrated that ELCA failed to achieve better clinical outcomes than balloon angioplasty while it increased the risk of vessel dissection and perforation from the formation of intraluminal vapor bubbles in blood. However, current ELCA techniques, particularly saline infusion, are different than those used in these early trials. Additionally, some applications of ELCA, such as saphenous vein grafts, in-stent restenosis, and CTOs, were not included in these trials.
Clinical Examples
Balloon undilatable and balloon-uncrossable lesions. ELCA can provide the debulking needed to increase the likelihood of success in moderately calcified lesions when passage of a RotaWire or a suitable microcatheter for wire exchange is not possible. This was tested with the 0.9 mm X80 catheter, which allows operation of higher energy (80 mJ/mm²; 80 Hz) compared with standard catheters (60 mJ/mm²; 40Hz) in 100 calcified and/or balloon-resistant lesions (n = 95 patients).19 The procedural success rate was 93% and clinical success rate was 86%, with a low rate of procedural complications (5 laser-related dissections and 1 non-Q wave MI).
ELCA provides an excellent tool for overcoming these difficult cases, with its advantage of being compatible with any PCI guidewire, even in the presence of another buddy wire. Even when the catheter does not pass through the lesion, lasing at the proximal area with increasing settings will often allow crossing of a balloon.
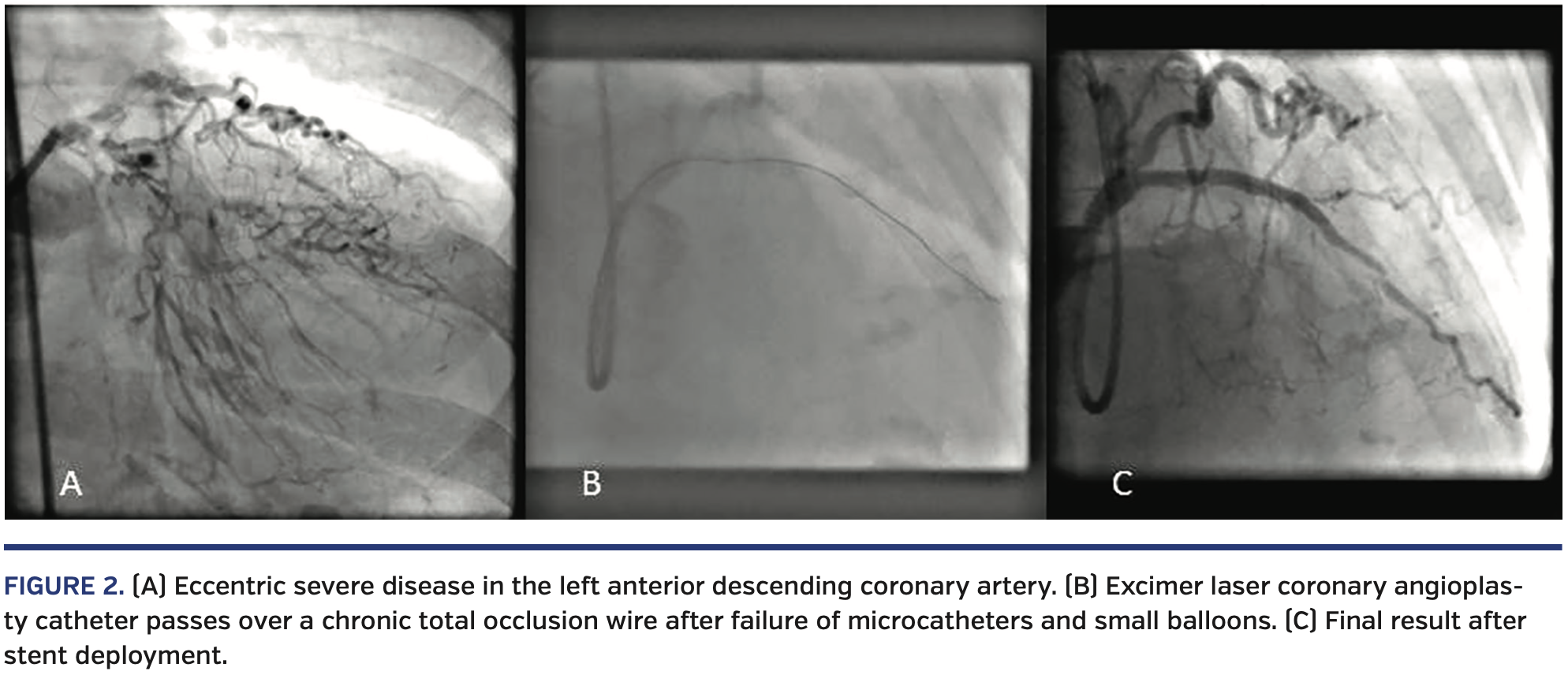
Figure 2 is a case of severely calcified and eccentric lesion in the left anterior descending coronary artery, which could not be crossed with a RotaWire (Boston Scientific) or a standard PCI wire and was only crossed with difficulty with a stiff CTO wire. However, a microcatheter would not pass for wire exchange with the RotaWire, and similarly the smallest PCI balloon would not pass despite using a GuideLiner extension catheter (Teleflex). A 0.9 mm laser catheter was used and a channel was created that allowed the passage of a balloon (and subsequently stents) with a successful outcome.
Under-expanded stents. Once deployed, it is often difficult to expand stents in under-prepared lesions (mainly due to fibrocalcific or calcified atheroma). Techniques such as cutting balloon and rotational atherectomy may be ineffective. The use of ELCA for ablation of tissue, and therefore debulking, can assist in balloon expansion within a vessel that has proven resistant to dilation after stent deployment.
ELCA is our current approach to under-expanded stents, and we have used it for the last few years with excellent results and success.12 The laser is used in the normal fashion in saline flush and with increasing energy and frequency, as required. We have described the successful use of concurrent contrast during laser for an old, under-expanded stent.12
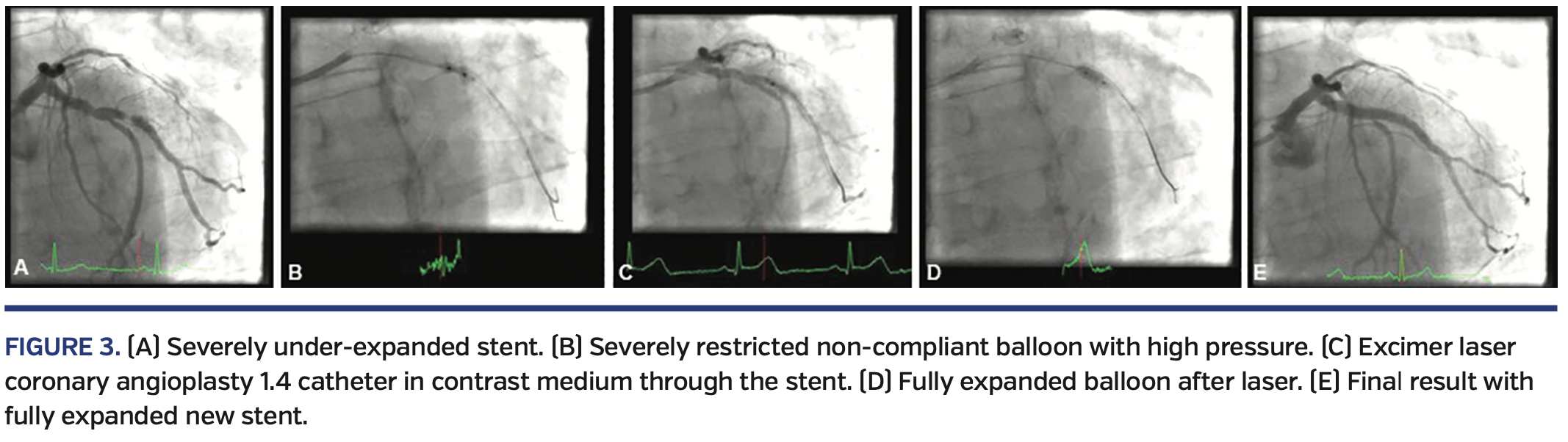
Figure 3 illustrates the case of a patient treated with a drug-eluting stent that was under-deployed and under-expanded despite using high-pressure postdilation up to 30 atm. He returned with severe symptoms and severe in-stent restenosis, which was undilatable with a high-pressure non-compliant balloon. A 1.4 mm laser catheter did not pass originally in saline medium, but then using contrast medium while lasing the restenosis was crossed and the stent expanded, and a new stent was inserted and postdilated with excellent results.
Chronic total occlusion. Figure 4 is an example of a CTO case of short occlusion at the proximal RCA, which was crossed with a Pilot 200 wire (Abbott) but could not be crossed with any low-profile balloons, Corsair microcatheter (Asahi Intecc), or Turnpike Gold microcatheter (Teleflex). A 0.9 mm laser catheter was used with diluted contrast medium and passed at 80/80 setting, allowing the successful completion of the procedure.
Recently, laser has been used in CTOs when it was difficult to puncture through the proximal cap; in these cases, the laser was used and activated in contrast medium, without an angioplasty wire, and resulted in cap modification, which allowed the passage of a CTO guidewire and the successful completion of the procedure (personal experience). This approach remains off label and should only be undertaken by experienced laser operators.
RASER-PCI. In cases of heavy calcification, where rotablation is the preferred approach but the rotablation wire is unable to pass, we have used the combination of laser and rotablation. First, a normal PCI wire is passed, and then the laser catheter is used to create a channel that allows the delivery of the thin rotablation wire to allow for maximum debulking of heavy calcium. We’ve term this procedure “RASER-PCI,” and it has allowed us to achieve a successful procedural outcome.13 As mentioned before, one of the advantages of laser is the ability to use it on standard PCI wires, in contrast to rotablation, which can only be performed on its special thin wire that is difficult to deliver in calcified and severe lesions. Novel atherectomy guidewires, such as the recently introduced ViperWire Advance flex tip (CSI), may facilitate primary lesion wiring.
Figure 5 illustrates a case where the rotablation wire could not be delivered distally and a normal PCI wire was used, but a microcatheter could not be delivered for exchange either. RASER-PCI was used with a 0.9 mm laser catheter, which created a channel. The rotablation wire was then delivered and rotablation was performed with a successful final outcome.
Saphenous vein graft. Laser may be useful for PCI of multifocal, diffuse, and thrombotic saphenous vein graft (SVG) lesions, potentially reducing the risk of distal embolization. In a small, retrospective study of 31 patients, Ebersole et al found that ELCA was associated with a high rate of success in acute occlusion of SVGs.20 Laser success rate was 87%, angiographic success rate was 97%, and overall procedural success rate was 84%. Non-Q wave MI (CK-MB increase post procedure) occurred in 2 patients (6%), in-hospital death occurred in 1 patient (3%), and repeat target-vessel intervention was necessary in another patient (3%). There were 3 significant dissections (10%; 2 were laser induced and 1 was guidewire induced). There were no episodes of acute closure or distal embolization. In one of the original laser registries, data were analyzed for 545 stenoses treated with ELCA.21 Distal embolization occurred in only 18 patients (3%), and ostial lesions and lesions within small SVGs had more favorable outcomes after treatment with ELCA. Based on very limited current data, ELCA may be used in SVGs when filters cannot be utilized.
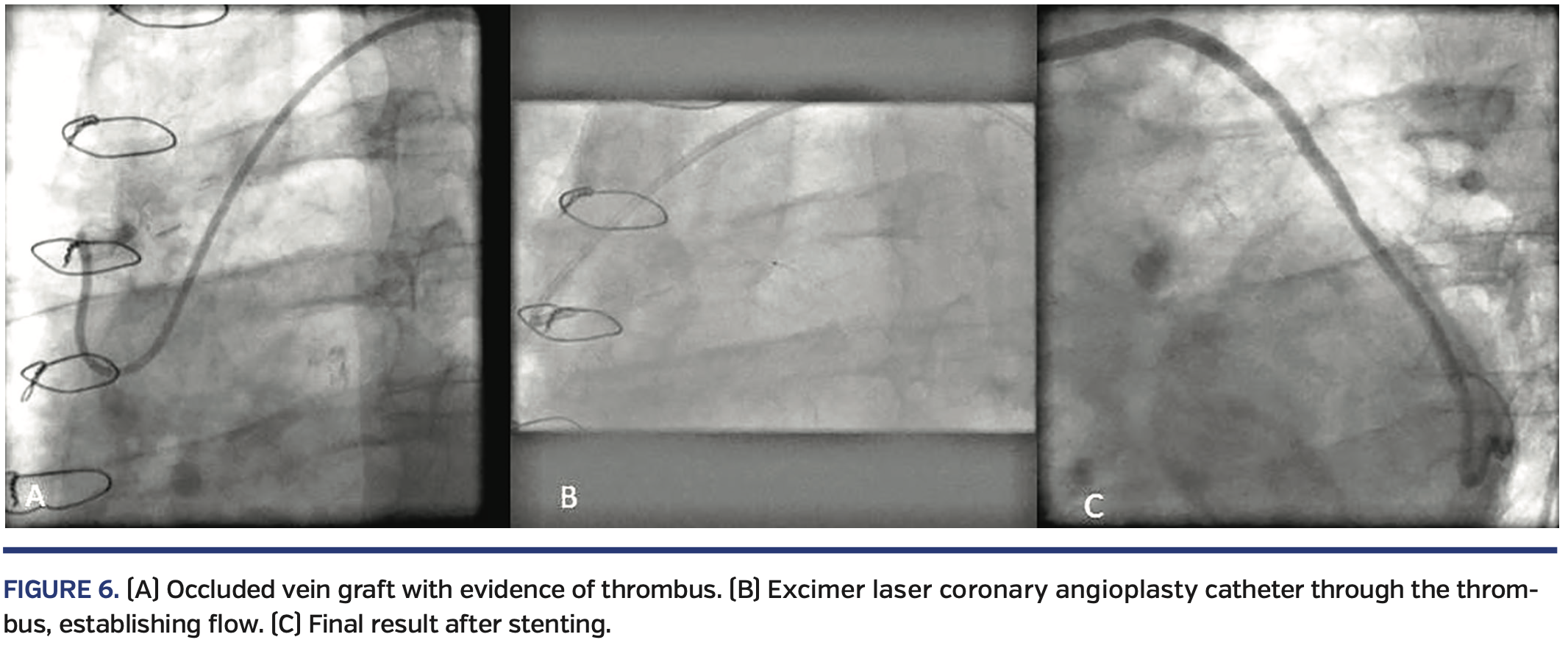
Figure 6 demonstrates an acutely occluded SVG wherein normal ballooning failed to establish any flow due to a heavy thrombus burden. Subsequent laser use established antegrade flow and allowed the successful completion of the procedure.
Future Directions
The use of ELCA has regained its momentum due to improved safety and performance with new, more-advanced catheters and the use of saline medium to deliver energy.
Work is being performed toward development of laser catheters that can be used to ablate through heavily calcified plaques. This will be a significant advance, as laser is currently not very effective in heavily calcified lesions.
Laser will remain an important tool for uncrossable, undilatable lesions, which is the most common current indication, with emerging uses in other clinical scenarios.
Laser use for under-expanded stents is a new approach for managing these difficult and clinically important cases. The use of laser with contrast in resistant lesions may also gain momentum, but should remain restricted to experienced laser users and to limited situations. In addition, saline flush should remain the standard practice.
Laser has also been used to modify and penetrate a resistant proximal CTO cap, which is important given advances in CTO-PCI. The combined use of laser and rotablation (RASER-PCI) is a useful, albeit expensive, approach for a subset of highly resistant and heavily calcified lesions.
Conclusion
Excimer laser debulks and modifies the tissue with its photochemical, photothermal, and photokinetic properties without causing significant injury. After a disappointing start, ELCA has undergone important refinements and advancements that have earned it a renewed place in treating complex and resistant coronary lesions.
When used as instructed by the manufacturer, ELCA is an important tool that allows the completion of difficult and complicated cases. It is a useful tool in the catheterization laboratory for treating lesions that are uncrossable or undilatable, a subset that is increasing with the aging population that we treat on a daily basis. ELCA is also helpful in cases where a stent was deployed but remains under-expanded, with accumulating evidence of its efficacy in this situation.
In addition, laser is increasingly used for CTO-PCI to facilitate the modification of the proximal CTO cap, to allow penetration with a wire and the completion of the procedure. Laser has been used in certain circumstances and by experienced operators in a contrast medium (rather than saline medium) to increase its effectiveness and allow the successful completion of complex PCI cases.
In comparison with rotablation, ELCA has the advantage of working with a normal 0.014˝ PCI guidewire and in the presence of a second buddy wire, which is frequently used in difficult lesions. Rotablation cannot be used with a second wire in place and requires a special 0.009˝ thin wire that is occasionally difficult to deliver.
Overall, ELCA is an important tool in the interventionalists’ armamentarium that, when used appropriately, allows the completion of difficult and demanding cases with successful outcomes, and therefore benefits our patients.
From 1the Department of Cardiology, Freeman Hospital, Newcastle upon Tyne, United Kingdom; Institute of Cellular Medicine, Newcastle University, Newcastle upon Tyne, United Kingdom; and 2Minneapolis Heart Institute, Abbott Northwestern Hospital; Minneapolis, Minnesota.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Egred is a proctor for ELCA and reports honoraria and speaker fees from Philips, Abbott Vascular, Boston Scientific, Vascular Perspectives, Biosensors, Biotronik, and AstraZeneca. Dr Brilakis reports consulting/speaker honoraria from Abbott Vascular, the American Heart Association (associate editor, Circulation), Biotronik, Boston Scientific, Cardiovascular Innovations Foundation (Board of Directors), CSI, Elsevier, InfraRedx, GE Healthcare, Siemens, Teleflex, and Medtronic; research support from Siemens, Regeneron, and Osprey; shareholder in MHI Ventures.
Manuscript submitted August 14, 2019, accepted August 20, 2019.
Address for correspondence: Mohaned Egred, BSc (Hons), MBChB, MD, FRCP, FESC, Cardiothoracic Department, Freeman Hospital, Newcastle University, Newcastle-Upon-Tyne, Tyne and Wear, NE7 7DN, United Kingdom. Email: m.egred@nuth.nhs.uk
- Waller BF. “Crackers, breakers, stretchers, drillers, scrapers, shavers, burners, welders and melters” — the future treatment of atherosclerotic coronary artery disease? A clinical-morphologic assessment. J Am Coll Cardiol. 1989;13:969-987.
- Cook SL, Eigler NL, Shefer A, Goldenberg T, Forrester JS, Litvack F. Percutaneous excimer laser coronary angioplasty of lesions not ideal for balloon angioplasty. Circulation. 1991;84:632-643.
- Appelman YE, Piek JJ, Strikwerda S, et al. Randomised trial of excimer laser angioplasty versus balloon angioplasty for treatment of obstructive coronary artery disease. Lancet. 1996;347:79-84.
- Reifart N, Vandormael M, Krajcar M, et al. Randomized comparison of angioplasty of complex coronary lesions at a single center. Excimer laser, rotational atherectomy, and balloon angioplasty comparison (ERBAC) study. Circulation. 1997;96:91-98.
- Choy DS, Stertzer S, Rotterdam HZ, Sharrock N, Kaminow IP. Transluminal laser catheter angioplasty. Am J Cardiol. 1982;50:1206-1208.
- Ginsburg R, Kim DS, Guthaner D, Toth J, Mitchell RS. Salvage of an ischemic limb by laser angioplasty: description of a new technique. Clin Cardiol. 1984;7:54-58.
- Grundfest WS, Litvack F, Forrester JS, et al. Laser ablation of human atherosclerotic plaque without adjacent tissue injury. J Am Coll Cardiol. 1985;5:929-933.
- Litvack F, Grundfest W, Eigler N, et al. Percutaneous excimer laser coronary angioplasty. Lancet. 1989;2:102-103.
- Bittl JA, Brinker JA, Sanborn TA, Isner JM, Tcheng JE. The changing profile of patient selection, procedural techniques, and outcomes in excimer laser coronary angioplasty. Participating investigators of the percutaneous excimer laser coronary angioplasty registry. J Interv Cardiol. 1995;8:653-660.
- Oesterle SN, Bittl JA, Leon MB, et al. Laser wire for crossing chronic total occlusions: “learning phase” results from the U.S. TOTAL trial. Total occlusion trial with angioplasty by using a laser wire. Cathet Cardiovasc Diagn. 1998;44:235-243.
- Serruys PW, Hamburger JN, Koolen JJ, et al. Total occlusion trial with angioplasty by using laser guidewire. The TOTAL trial. Eur Heart J. 2000;21:1797-1805.
- Egred M. A novel approach for under-expanded stent: excimer laser in contrast medium. J Invasive Cardiol. 2012;24:E161-E163.
- Egred M. RASER angioplasty. Catheter Cadriovasc Interv. 2012;79:1009-1012.
- Deckelbaum LI, Natarajan MK, Bittl JA, et al. Effect of intracoronary saline infusion on dissection during excimer laser coronary angioplasty: a randomized trial. The percutaneous excimer laser coronary angioplasty (PELCA) investigators. J Am Coll Cardiol. 1995;26:1264-1269.
- Ghazzal ZM, Hearn JA, Litvack F, et al. Morphological predictors of acute complications after percutaneous excimer laser coronary angioplasty. Results of a comprehensive angiographic analysis: importance of the eccentricity index. Circulation. 1992;86:820-827.
- Litvack F, Eigler N, Margolis J, et al. Percutaneous excimer laser coronary angioplasty: results in the first consecutive 3,000 patients. The ELCA investigators. J Am Coll Cardiol. 1994;23:323-329.
- Holmes DR Jr, Mehta S, George CJ, et al. Excimer laser coronary angioplasty: the new approaches to coronary intervention (NACI) experience. Am J Cardiol. 1997;80:99K-105K.
- Vandormael M, Reifart M, Preusler W, et al. Six months follow-up results following excimer laser angioplasty, rotational atherectomy and balloon angioplasty for complex lesions: ERBAC study. Circulation. 1994;90:I-213A.
- Bilodeau L, Fretz EB, Taeymans Y, Koolen J, Taylor K, Hilton DJ. Novel use of a high-energy excimer laser catheter for calcified and complex coronary artery lesions. Catheter Cardiovasc Interv. 2004;62:155-161.
- Ebersole D, Dahm JB, Das T, et al. Excimer laser revascularization of saphenous vein grafts in acute myocardial infarction. J Invasive Cardiol. 2004;16:177-180.
- Bittl JA, Sanborn TA, Yardley DE, et al. Predictors of outcome of percutaneous excimer laser coronary angioplasty of saphenous vein bypass graft lesions. The percutaneous excimer laser coronary angioplasty registry. Am J Cardiol. 1994;74:144-148.