Evaluating Embolic Reduction Techniques Concurrent to Infrainguinal Interventions: A Single-Center Experience
Abstract: Objective. To report a single-center retrospective evaluation of embolic reduction strategies concurrent to infrainguinal atherectomy intervention. Methods. Fifty-five consecutive atherectomy patients from 2011 to 2012 treated with embolic reduction devices were analyzed. Embolic load was stratified by lesion type, atherectomy technique, debris capture effectiveness, and risk factor characteristics. Over 80% of lesions were complex. Baseline stenosis and average lesion length were 94.2 ± 6.4% and 172.8 ± 85.5 mm, respectively. Our embolic reduction strategy included a systematic approach using the Proteus device in all cases and then in combination with the SpiderFX for long or complex lesions. Debris was analyzed for count and aggregate surface area. Results. Procedural success was 98.2%, with no in-hospital serious adverse events. The mean aggregated surface area of captured debris was 22 ± 20 mm2. Thrombolysis with laser produced the highest amount of embolic load followed by laser, directional, and orbital atherectomy procedures (P<.05). Patients with restenotic/in-stent restenotic lesions produced larger amounts of debris (P<.05). No embolizations were recorded up to discharge. Proteus accounted for two-thirds of the debris captured in our study; its capture efficiency increased as device and lesion length met. An inverse relation was also found between lesion length and embolic protection device capture efficacy (TASC-II B vs D; P<.02). Conclusion. An embolic reduction strategy using the Proteus catheter alone, particularly when Proteus and lesion lengths meet, or with the SpiderFX in complex infrainguinal atherectomy procedures, can be an effective tool. Current findings suggest potential optimization of future “at risk” interventions.
J INVASIVE CARDIOL 2014;26(6):277-282
Key words: lower limbs, Proteus, embolic protection devices, peripheral, distal embolization, atherectomy
_________________________________
Peripheral vascular intervention (PVI) represents a serious and established concern to peripheral circulation by exposing patients to embolic risks, which can consequently require re-interventions such as thrombectomy or thrombolysis.1 Some patient groups, especially those with critical limb ischemia (CLI), are already predisposed to embolic risks and present a serious healthcare problem.2
Despite recent findings, lower-limb distal embolization (DE) and its clinical ramifications during infrainguinal settings is a source for debate and controversy among interventionalists.1-16 The incidence of symptomatic emboli ranges from 2.4%-30% and reaches even higher rates following atherectomy or thrombolytic declotting attempts.1,2,4-6 DE requiring further treatment occurs in 2%-5% of unselected infrainguinal patients and is largely dependent on the type of patient, lesion, and procedure performed, with significant variations between groups.8,9,13,14 Frequent occurrences of DE in complex, long (TASC II C/D), or thrombotic lesions are well documented in previous studies.1,10,11 Davies et al reported that 26.5% and 29.7% of patients with DE suffered from either deterioration or no change in symptoms following endovascular intervention, respectively; the control group incidence was 0.4% and 14.1%, respectively (P<.001).12
Embolic load (EL) is expected to be higher in the presence of the predisposing factors mentioned above. However, lack of consistent evidence, ease of use, and economic factors16 render the routine use of EL reduction devices in real-world PVIs uncommon. Shammas noted that the efficiency of debris capture with the Proteus device can be assessed when used in conjunction with an embolic protection device (EPD).9 We considered this approach during our evaluation of embolic reduction strategies, since both devices are routinely used in our center. We share our single-center experience using the Proteus embolic capture balloon for PVI cases and in combination with the SpiderFX for more complex (TASC II D) lesions.
Methods
Study design. Data from this single-arm, single-center retrospective study were collected from 55 unselected, consecutive, lower-extremity atherectomy interventions conducted between November 2011 and November 2012. The data went through statistical analysis to assess common embolic reduction devices implemented at our center.
Patient sample. Baseline data (Table 1) were collected (28 males; mean age, 70.6 years; age range, 50-87 years). Seven subjects presented with open and non-healing wounds, 12 had severe ischemic rest pains, 33 had severe claudication, and 3 had moderate claudication. There were 29 TASC II D lesions, 16 TASC II C lesions, and 10 TASC II B lesions; 24 de novo lesions, 27 restenotic lesions, and 10 in-stent restenotic lesions; 20 in-stent re-occlusions, 22 thrombotic occlusions, and 22 chronic total occlusions. Baseline stenosis and average lesion length were 94.24 ± 6.4% and 172.8 ± 85.5 mm, respectively. Forty-one subjects had none to mild calcification, 6 had moderate calcification, and 8 had severe calcifications.
Study devices. The Proteus embolic capture angioplasty balloon (Angioslide, Ltd) and the SpiderFX EPD (ev3/Covidien) were used in the study. The Proteus has a dual function of performing angioplasty and removing embolic debris. The device is a 0.035/0.014˝ over-the-wire dual-lumen catheter with a foldable semicompliant balloon, a working length of 135 cm and 5.5/4 Fr crossing profile. Post angioplasty, embolic capture is activated by deflating the balloon to 2 atm (from nominal 8 atm) and folding it inward tip-side first, toward the proximal end of the balloon with the use of a pulling knob. Upon deflation, the negative pressure captures embolic debris within the balloon cavity, which is removed during retrieval of the device.17,18
The SpiderFX EPD is a single-hoop, nitinol braid filter. It is 0.014˝/0.018˝ guidewire compatible and is deployed and recovered through a dual-ended and low-profile catheter compatible with a 6 Fr guide catheter or 5 Fr sheath.
Endovascular procedure. All patients underwent dual-antiplatelet therapy and received intravenous heparin (70 U/kg) prior to the intervention per standard department protocol. Directional  atherectomy with the SilverHawk/TurboHawk device (eV3/Covidien) was performed in 21 cases (38.2%); laser atherectomy (Spectranetics) was performed in 19 cases (34.5%), and orbital atherectomy (Cardiovascular Systems, Inc) was performed in 7 cases (12.7%). In 30 cases (55%), the SpiderFX EPD was used in addition to the Proteus device. Use of the SpiderFX was determined by the physician; it was used in long or complex lesions (11/26 TASC II B/C lesions [42%] and 20/29 TASC II D lesions [67%]). Lesions were treated with atherectomy based on department protocols and discretion of the primary physician. In 8 cases (14.5%), pharmacological therapy administered locally through the Clearway System (Atrium) was required as an adjacent therapy to laser and was registered as a separate procedure. The Proteus balloon was used for postatherectomy dilation and removal of debris in all cases (Table 2), with an average of 1.2 devices per vessel.
atherectomy with the SilverHawk/TurboHawk device (eV3/Covidien) was performed in 21 cases (38.2%); laser atherectomy (Spectranetics) was performed in 19 cases (34.5%), and orbital atherectomy (Cardiovascular Systems, Inc) was performed in 7 cases (12.7%). In 30 cases (55%), the SpiderFX EPD was used in addition to the Proteus device. Use of the SpiderFX was determined by the physician; it was used in long or complex lesions (11/26 TASC II B/C lesions [42%] and 20/29 TASC II D lesions [67%]). Lesions were treated with atherectomy based on department protocols and discretion of the primary physician. In 8 cases (14.5%), pharmacological therapy administered locally through the Clearway System (Atrium) was required as an adjacent therapy to laser and was registered as a separate procedure. The Proteus balloon was used for postatherectomy dilation and removal of debris in all cases (Table 2), with an average of 1.2 devices per vessel.
PTA balloon catheters were used adjunctively in 11 procedures (20%). There were 7 stent procedures, 2 due to vessel dissections and 5 due to suboptimal results. The average number of stents per 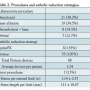 limb and stent length were 1.14 ± 0.37 and 111 ± 18.07 mm, respectively. At the end of the procedure, patency rates were achieved through digital subtraction angiography of the target vessel in two planes and distal angiograms were compared for detection of embolic events. Patients were monitored postoperatively up to discharge and followed for 30 days. After the initial 30 days, patients were followed periodically during the first year.
limb and stent length were 1.14 ± 0.37 and 111 ± 18.07 mm, respectively. At the end of the procedure, patency rates were achieved through digital subtraction angiography of the target vessel in two planes and distal angiograms were compared for detection of embolic events. Patients were monitored postoperatively up to discharge and followed for 30 days. After the initial 30 days, patients were followed periodically during the first year.
Technical success was defined as the ability to deploy and retrieve the devices. Procedural success was defined as a residual narrowing of <30% without a flow-limiting dissection or DE requiring further mechanical or pharmacological treatment. Major adverse event was defined as DE in hospital, DE at 30 days, flow-limiting dissection, death, amputation, major access-site complications, or revascularization.
Embolic load analysis. Prior to disposal, removed particles from both the Proteus and SpiderFX devices were analyzed outside the sterile field using the Proteus Particle Visualization Kit (PVK) provided with each Proteus catheter. Debris were flushed, stained with violet marking dye (Bradley Products, Inc), and collected in a strainer basket (40 µm). Strainers were then placed on a scale for documentation, followed by image analysis using Photoshop CS3 (Version 10.1, Adobe) and Fiji (Image J 1.45b with add-ons; Washington DC). The pixel size was converted to metric dimensions (mm) and Fiji’s Analyze Particles function was utilized to determine the overall count, major axial dimension, and surface area of each particle.17 Recovered embolic material was not histologically evaluated.
Statistics. Statistical analysis was performed with SAS version 9.3 (SAS Institute, Inc). Study data are presented in tables and figures, where discrete data are presented as numbers and percentages. Continuous data are presented as either mean ± standard deviation or median with range. The analyses were performed for a set of consecutive atherectomy patients and compared for the EL (1) in various lesion types and (2) in different atherectomy techniques. We also evaluated (3) stratification per risk factors and (4) debris capture effectiveness. Single groups were compared with the Wilcoxon two-sample test. Groups greater than two were compared with the Kruskal-Wallis test. Similarities between groups were evaluated using t-tests and 95% overlapping confidence intervals. All tests were two sided and the threshold of statistical significance was set at P<.05. Nominal values are presented.
Results
Technical success rates for both the Proteus and SpiderFX devices were 100%. Final patency was 83.73 ± 7.4%. Procedural success of ≤30% residual stenosis was achieved in 54/55 cases performed (98.18%). Forty-five of 55 patients (82%) were followed for a mean period of 60 days. Three patients (5.45%) underwent same-limb reintervention at 4-9 months.
Safety. One patient died, from cardiac arrest, within a month following the intervention due to preexisting conditions and severe cardiomyopathy. There were 2 cases of flow-limiting dissection that were detected and treated by provisional stenting during the procedure. All patients were monitored and showed neither signs nor symptoms of DE or any other type of complication up to discharge. 
Embolic load analysis. Total EL was divided between SpiderFX and Proteus, such that the majority of particles were captured by the latter (P<.001), which accounts for two-thirds of the debris in terms of aggregate particle surface area. A similar distribution was found for the various subgroups of lesions analyzed (Table 3 and Figures 1 and 2). While sample size was small, the Proteus capture efficiency increased as balloon and lesion lengths met (85.5 ± 12.7% vs 64.0 ± 22.0% TASC II B vs TASC II D; P<.02). The overall Proteus capture efficiency was unrelated to EPD deployment during the procedure (28.32 ± 21.70 mm2 [95% confidence interval (CI), 15.8-40.8] and 36.07 ± 23.42 mm2 [95% CI, 18.1- 54.1], respectively).
Atherectomy procedures conducted on restenotic and ISR/reoccluded lesions produced more than double the number of particles and aggregate surface area (EL) than de novo lesions (42.53 ± 21.42 mm2 vs 21.42 ± 15.09 mm2; P<.01). ISR lesions produced the greatest amount of embolic load over fewer particles counted (46.90 ± 35.77 mm2; P<.02) followed by restenotic lesions (44.96 ± 33.07 mm2). Occlusions produced about a 50% higher embolic load when compared with high-grade stenotic (≥75%) lesions (39.80 ± 31.08 mm2 vs 26.14 ± 21.22 mm2; P<.08). TASC II D lesions produced approximately more than double the amount of debris (aggregate surface area) than TASC II B/C lesions, spread between 70% and 122% more particles, respectively (P<.02).
approximately more than double the amount of debris (aggregate surface area) than TASC II B/C lesions, spread between 70% and 122% more particles, respectively (P<.02).
In regard to procedure type, thrombolysis (combined with laser atherectomy) was found to produce the highest amount of total embolic load, followed by laser, directional, and orbital atherectomy procedures, respectively (59.84 ± 36.88 mm2, 36.99 ± 31.36 mm2, 27.33 ± 17.20 mm2, and 17.04 ± 11.14 mm2; P<.05). The Proteus device captured all recorded debris, since SpiderFX cannot be used with orbital atherectomy due to technical limitations.
Stratification by risk factors reveals interesting trends. Larger amounts of DE debris were predisposed during the intervention of restenotic or ISR lesions compared to de novo lesions in current smokers (P<.01), patients with diabetes mellitus (P<.01), hyperlipidemia (P<.01), and obesity (P<.03).
Discussion
Lack of consistent evidence, ease of use, and economic factors16 render the routine use of EL reduction strategies in real-world PVI’s uncommon. As both the Proteus and SpiderFX are routinely used in our center, our aim was to evaluate a systematic approach of when to consider embolic reduction strategies in complex infrainguinal procedures. The EL in this study was found to be material in all lesion types. Differences exist between the various subgroups, creating identifying factors that predispose for higher embolic load.1,8-10,13,14 Occlusive, long, restenotic/ISR, and thrombotic lesions, along with thrombolysis, laser, directional, and orbital procedures, were all found to be major distal EL producers. Our findings support the rationale to consider an embolic reduction strategy using a systematic approach to minimize EL.
in our center, our aim was to evaluate a systematic approach of when to consider embolic reduction strategies in complex infrainguinal procedures. The EL in this study was found to be material in all lesion types. Differences exist between the various subgroups, creating identifying factors that predispose for higher embolic load.1,8-10,13,14 Occlusive, long, restenotic/ISR, and thrombotic lesions, along with thrombolysis, laser, directional, and orbital procedures, were all found to be major distal EL producers. Our findings support the rationale to consider an embolic reduction strategy using a systematic approach to minimize EL.
The Proteus captured a heavier EL over previously reported literature.10 In addition, distribution of debris between the SpiderFX and Proteus devices unveils their distinctive capturing characteristics. These differences are attributed to several factors: (1) technological approach, ie, strainer (EPD filter) vs active suction (Proteus); (2) anatomical location, ie, the former being placed distally to the latter; and (3) time of activity, ie, the SpiderFX is used throughout the procedure, whereas Proteus is used during postatherectomy angioplasty.
Davies et al reported that smoking status and calcification influenced the amount of DE, while lesion complexity influenced limb salvage rates.12 Our findings illustrate the importance of subgroup analysis, by which restenotic and ISR lesions released significantly heavier EL in the presence of associated risk factors such as diabetes mellitus, smoking, hyperlipidemia, and obesity.
Average lesion length was 172.8 ± 85.5 mm while the available United States Proteus device length is limited to 100 mm, which serves as its primary caveat. Proteus accounted for two-thirds of the debris captured in our study; its capture efficiency was found to increase as device and lesion length met. An inverse relation was also found between lesion length and EPD capture efficacy (TASC II B vs TASC II D; P<.02). We suggest a systematic approach when considering embolic reduction strategies by using the Proteus device in at-risk patients and procedures such as single-vessel run-off, and ISR/reocclusions with TASC II B/C lesions. When the embolic load is expected to be heavier, such as in the case of TASC II D lesions and lytic therapy, the combination of the Proteus and an EPD should be considered.
Study limitations. This study had many limitations due to its size and design and is based on a single-center retrospective review of collected data. While our findings may suggest future optimization of PVIs, embolic reduction techniques need to be further investigated in larger cohorts with regard to mid- and long-term costs and clinical values. Future studies may investigate a combination vs single reduction strategy over separate cohorts as well as the efficacy of longer Proteus devices.
Conclusions
Based on our single-center experience, we found a systematic embolic reduction strategy may provide additional benefit in preventing liberated debris from threatening blood supply to the distal limb. This was shown to be an effective measure for capturing and removing embolic debris across all clinical conditions and procedure types. Physicians aiming to use EL management strategies may consider using Proteus in at-risk patients and procedures such as single-vessel run-off and ISR/reocclusions with TASC II B/C lesions. When the embolic load is expected to be heavier, such as TASC II D lesions and lytic therapy, the combination of the Proteus and an EPD should be considered. Further research is required in order to determine the long-term clinical benefit of embolic reduction strategies in prospective, randomized studies.
References
- Shammas NW, Shammas GA, Dippel EJ, Jerin M, Shammas WJ. Predictors of distal embolization in peripheral percutaneous interventions: a report from a large peripheral vascular registry. J Invasive Cardiol. 2009;21(12):628-631.
- Shammas NW, Dippel EJ, Coiner D, Shammas GA, Jerin M, Kumar A. Preventing lower extremity distal embolization using embolic filter protection: results of the PROTECT registry. J Endovasc Ther. 2008;15(3):270-276.
- Lam RC, Shah S, Faries PL, McKinsey JF, Kent KC, Morrissey NJ. Incidence and clinical significance of distal embolization during percutaneous interventions involving the superficial femoral artery. J Vasc Surg. 2007;46(6):1155-1159.
- Kaid KA, Gopinathapillai R, Qian F, Salvaji M, Wasty N, Cohen M. Analysis of particulate debris after superficial femoral artery atherectomy. J Invasive Cardiol. 2009;21(1):7-10.
- Chalmers RT, Hoballah JJ, Kresowik TF, et al. Late results of a prospective study of direct intra-arterial urokinase infusion for peripheral arterial and bypass graft occlusions. Cardiovasc Surg. 1995;3(3):293-297.
- Wholey MH, Maynar MA, Wholey MH, et al .Comparison of thrombolytic therapy of lower-extremity acute, subacute, and chronic arterial occlusions. Cathet Cardiovasc Diagn. 1998;44(2):159-169.
- Thompson MM, Smith J, Naylor AR, et al. Microembolization during endovascular and conventional aneurysm repair. J Vasc Surg. 1997;25(1):179-186.
- Shrikhande GV, Khan SZ, Hussain HG, Dayal R, McKinsey JF, Morrissey N. Lesion types and device characteristics that predict distal embolization during percutaneous lower extremity interventions. J Vasc Surg. 2011;53(2):347-352.
- Shammas NW. Balloon angioplasty with built-in embolic protection mechanism: the dual role of the proteus balloon. J Endovasc Ther. 2012;19(5):617-619.
- Karnabatidis D, Katsanos K, Kagadis GC, et al. Distal embolism during percutaneous revascularization of infra-aortic arterial occlusive disease: an underestimated phenomenon. J Endovasc Ther. 2006;13:269-280.
- Axisa B, Fishwick G, Bolia A, et al. Complications following peripheral angioplasty. Ann R Coll Surg Engl. 2002;84(1):39-42.
- Davies MG, Bismuth J, Saad WE, et al. Implications of in situ thrombosis and distal embolization during superficial femoral artery endoluminal intervention. Ann Vasc Surg. 2010;24(1):14-22.
- McDermott JC, Crummy AB, Voegeli DR, Starck EE. Complications of transluminal angioplasty. Radiology. 1987;162(1 Pt 1):286-288.
- Becker GJ, Katzen BT, Dake MD. Noncoronary angioplasty. Radiology. 1989;170(3 Pt 2):921-940.
- Al-Hamali S, Baskerville P, Fraser S, Walters H, Markus HS. Detection of distal emboli in patients with peripheral arterial stenosis before and after iliac angioplasty: a prospective study. J Vasc Surg. 1999;29(2):345-351.
- Wholey M. The role of embolic protection in peripheral arterial atherectomy. Tech Vasc Interv Radiol. 2011;14(2):65-74.
- Makam P. Capture and removal of atheromatous plaque, from the lesion site, post directional atherectomy of long, in-stent restenotic superficial femoral artery lesions. J Invasive Cardiol. 2013;25(3):E63-E65.
- Zeller T, Schmidt A, Rastan A, Noory E, Sixt S, Scheinert D. Initial experience with the 5 × 300-mm proteus embolic capture angioplasty balloon in the treatment of peripheral vascular disease. J Endovasc Ther. 2012;19(6):826-833.
- Shammas NW, Coiner D, Shammas GA, Christensen L, Dippel EJ, Jerin M. Distal embolic event protection using excimer laser ablation in peripheral vascular interventions: results of the DEEP EMBOLI registry. J Endovasc Ther. 2009;16(2):197-202.
- Hadidi OF, Mohammad A, Zankar A, Brilakis ES, Banerjee S. Embolic capture angioplasty in peripheral artery interventions. J Endovasc Ther. 2012;19(5):611-616.
________________________________
From Community Health Care System, Munster, Indiana.
Disclosure: The author has completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Makam reports that he is a paid consultant, investor and on the Speaker’s Bureau for Cardiovascular Systems, Inc, is a paid consultant and a trainer for Covidien and Spectranetics, and was also a trainer for Angioslide, Ltd in 2012.
Manuscript submitted July 29, 2013, provisional acceptance given September 23, 2013, final version accepted December 4, 2013.
Address for correspondence: Prakash Makam, MD, Cardiology Associates of Northwest Indiana, P.C./10010 Donald S. Powers Drive, Munster, IN 46321. Email: prakashmakam@comcast.net












