The Efficacy and Safety of PRO-Kinetic Metal Alloy Stent in Hospitalized Patients with Acute ST-Elevation Myocardial Infarction (The PROMETHEUS Study)
Abstract: Background. We evaluated the clinical and angiographic outcomes of silicon carbide-coated cobalt chromium PRO-Kinetic bare-metal stent in patients with acute ST-segment elevation myocardial infarction (STEMI) who underwent primary percutaneous coronary intervention (PCI). Methods. Patients with acute STEMI (2.75-5.00 mm vessels; lesion length ≤30 mm by visual estimation) were treated with PRO-Kinetic stents at 5 centers in Korea. The primary endpoint was the rate of major adverse cardiac events (MACE), defined as all-cause death, new myocardial infarction, and target lesion revascularization (TLR) at 6-month follow-up. Follow-up angiography was recommended after the completion of the 6-month clinical follow-up. Results. A total of 64 patients (56.6 ± 12.9 years old, 56 male) were enrolled in the study. Procedural success was achieved in 100% of the lesions. The mean stent size was 3.51 ± 0.67 mm and the mean stent length was 20.3 ± 4.4 mm. There was 1 case of in-hospital death due to cardiac tamponade. During the 6-month clinical follow-up, 4 patients (6.3%) received TLR. Therefore, the total rate of MACE was 7.8%. Angiographic follow-up data were available for 42 patients (65.6%) and the in-stent late lumen loss was 1.02 ± 0.62 mm and in-segment late lumen loss was 0.99 ± 0.64 mm. Binary restenosis occurred in 53% of reference vessel diameters (RVDs) ≤3.0 mm, 25% of RVDs between 3.0 and 3.5 mm, and 0% of RVDs >3.5 mm (P=.006). Conclusions. The use of the PRO-Kinetic stent seems to be safe and feasible in primary PCI for acute STEMI, and shows favorable clinical and angiographic outcomes in large (>3.0 mm) coronary arteries, but not in small arteries.
J INVASIVE CARDIOL 2012;24(6):270-273
Key words: bare-metal stent, acute myocardial infarction, restenosis
_________________________________________________
Drug-eluting stents (DESs) have shown favorable results for the rates of restenosis and target lesion revascularization (TLR) compared with bare-metal stents (BMS) in patients with coronary artery disease.1,2 However, DESs may be associated with an increased incidence of stent thrombosis (ST), particularly late or very late ST.3-5 Delayed arterial healing processes, incomplete endothelial cell covering, inflammation, and hypersensitivity reaction of polymers may play an important role in ST.6
Silicon carbide is a coating material with anti-thrombogenic as well as anti-inflammatory effects, and silicon carbide coating was shown in vitro to reduce the thrombogenic properties of the metal surface and facilitate complete endothelialization.7,8 The Tenax for the Prevention of Restenosis and Acute Thrombotic Complications: A Useful Stent Trial (TRUST) study demonstrated the clinical safety and feasibility of silicon carbide-coated stents in unstable angina patients.9 However, there have been few studies about the safety and feasibility of silicon-carbide coated stents in primary percutaneous coronary intervention (PCI) for patients with acute ST-segment elevation myocardial infarction (STEMI).
The aim of this study was to evaluate the clinical and angiographic outcomes of silicon carbide passive-coated cobalt chromium stents (PRO-Kinetic coronary stent with PROBIO coating, Biotronik AG) in patients with STEMI.
Methods
Patients. The Efficacy and Safety of PRO-Kinetic Metal Alloy Stent in Hospitalized Patients with Acute ST-Elevation Myocardial Infarction (PROMETHEUS) study was performed in 5 primary PCI centers in Korea, with a prospective, open-label, and single-arm cohort design. The study protocol was reviewed and approved by the ethics committee of each hospital, and informed consent was obtained from each patient.
Patients were eligible for the study if they were >18 and <85 years old, had symptoms of STEMI for >30 minutes but ≤12 hours, and the electrocardiogram showed ST-segment elevation of more than 0.1 mV in 2 contiguous leads. The infarct-related vessel had to be a native coronary artery with a visually estimated reference vessel diameter (RVD) of 2.75-5.0 mm and lesion length ≤30 mm.
Study procedure and stent implantation. All patients received aspirin (300 mg loading and 100 mg/day indefinitely) and clopidogrel (300 mg loading and 75 mg/day for at least 1 month). Use of thrombectomy devices, predilatation, post-stenting adjunctive balloon inflation, intravascular ultrasound, intra-arterial balloon counterpulsation, or glycoprotein IIb/IIIa inhibitor were all left to operator discretion. Heparin was administered before PCI to keep activated clotting time at 250-300 seconds. Procedural success was defined as thrombolysis in myocardial infarction (TIMI) flow more than grade 2 with a final residual stenosis <30%.
Study endpoints. The primary endpoint was the rate of major adverse cardiac events (MACE), defined as all-cause death, new-onset MI, and TLR at 6-month follow-up exam. The secondary endpoint was defined as late lumen loss at the follow-up coronary angiogram. For this purpose, quantitative coronary angiographic (QCA) substudy was performed and routine follow-up angiography was recommended after the completion of the 6-month clinical follow-up. Stent thrombosis (ST) was defined according to Academic Research Consortium (ARC) definition.10
QCA analysis. Baseline, immediate postprocedure, and follow-up coronary angiographies were analyzed by QCA using the Cardiovascular Angiography Analysis System (CAAS) II (Pie Medical Imaging) by an experienced technician who was not aware of the study purpose.11
The minimum lumen diameter (MLD), RVD, percent stenosis, acute gain (defined as the difference of MLD before and after stent implantation), late lumen loss (defined as the difference between the postprocedure and follow-up MLD), and binary restenosis (defined as stenosis of 50% or more at follow-up angiography) were measured. All QCA measurements of the target lesion were obtained in the in-stent zone, and over entire segment including the stent and its 5 mm proximal and distal margins (in-segment zone).
Statistical analysis. Continuous variables were presented as mean ± standard deviation. Categorical variables, presented as frequencies and percentages, were compared using the Chi-square test or Fisher’s exact test as appropriate. All statistical analyses were performed with the aid of commercially available software (SPSS version 17.0; SPSS, Inc.).
Results
Patient demographics. A total of 64 patients were enrolled for the study. The mean age of the patients was 56.6 ± 12.9 years, and there were 56 males (87.5%). Eleven patients (17.2%) had a history of diabetes mellitus and 28 patients (43.8%) had a history of hypertension. The baseline characteristics are summarized in Table 1.
 Angiographic findings and procedural characteristics. Ten patients (15.6%) had 3-vessel disease, 20 patients (31.3%) had 2-vessel disease and 34 patients (53.1%) had 1-vessel disease. The right coronary artery (n = 30; 46.9%) was the most common target vessel and 41 lesions (64.1%) were of type B2 or C. The baseline angiographic and lesion characteristics of the patients are summarized in Table 2.
Angiographic findings and procedural characteristics. Ten patients (15.6%) had 3-vessel disease, 20 patients (31.3%) had 2-vessel disease and 34 patients (53.1%) had 1-vessel disease. The right coronary artery (n = 30; 46.9%) was the most common target vessel and 41 lesions (64.1%) were of type B2 or C. The baseline angiographic and lesion characteristics of the patients are summarized in Table 2.
The mean stent diameter was 3.51 ± 0.67 mm and the mean stent length was 20.3 ± 4.4 mm. Procedural success was achieved in 100% of the lesions. The preprocedure TIMI flow was 0 or 1 in 33 patients (51.6%) and postprocedure TIMI flow was 2 or 3 in all patients.
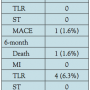 Clinical follow-up. There was 1 case of in-hospital death due to cardiac tamponade. During a 6-month clinical follow-up period, 5 patients underwent repeated coronary angiography due to the onset of new symptoms, positive stress test, or additional planned interventional procedures, of whom 4 (6.3%) received TLR. Therefore, the total rate of MACE was 7.8%. There was no episode of ST during follow-up (Table 3).
Clinical follow-up. There was 1 case of in-hospital death due to cardiac tamponade. During a 6-month clinical follow-up period, 5 patients underwent repeated coronary angiography due to the onset of new symptoms, positive stress test, or additional planned interventional procedures, of whom 4 (6.3%) received TLR. Therefore, the total rate of MACE was 7.8%. There was no episode of ST during follow-up (Table 3).
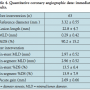 QCA data. Baseline QCA data were available for 63 patients. Eight patients had RVDs of less than 2.75 mm. The mean RVD was 3.32 ± 0.55 mm and average lesion length was 22.8 ± 4.7 mm. Postprocedural acute gain was 2.69 ± 0.66 mm (Table 4).
QCA data. Baseline QCA data were available for 63 patients. Eight patients had RVDs of less than 2.75 mm. The mean RVD was 3.32 ± 0.55 mm and average lesion length was 22.8 ± 4.7 mm. Postprocedural acute gain was 2.69 ± 0.66 mm (Table 4).
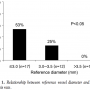
Follow-up QCA data were available for 42 patients; 5 before the completion of a 6-month clinical follow-up and 37 after a 6-month follow-up. In-stent late lumen  loss was 1.02 ± 0.62 mm and in-segment late lumen loss was 0.99 ± 0.64 mm (Table 5). Binary restenosis occurred in 53% of RVDs ≤3.0 mm, 25% of RVDs between 3.0 and 3.5 mm, and 0% of RVDs >3.5 mm (P=.006, Figure 1).
loss was 1.02 ± 0.62 mm and in-segment late lumen loss was 0.99 ± 0.64 mm (Table 5). Binary restenosis occurred in 53% of RVDs ≤3.0 mm, 25% of RVDs between 3.0 and 3.5 mm, and 0% of RVDs >3.5 mm (P=.006, Figure 1).
Discussion
This study is the first prospective clinical study evaluating the effects of PRO-Kinetic stents in patients with STEMI. In this study, we evaluated the 6-month clinical outcomes of the PRO-Kinetic stent, which showed low rates of TLR (6.3%) and MACE (7.8%), and no episodes of ST.
 DESs are now preferred in most PCI procedures because they reduce the incidence of restenosis and TLR. Most recent trials suggested that there were benefits to DESs relative to BMSs even in patients with acute MI.12-17 While DESs were obviously effective in reducing restenosis rates by inhibiting neointimal growth, data from a number of studies had shown conflicting results about their efficacy in the treatment of large coronary arteries.18-23 The TAXUS-IV analysis reported that there were no statistically significant benefits to DES in reducing the restenosis rate in patients with coronary arteries larger than 3.0 mm.20 The TAXUS-V study also showed that the frequency of 9-month adverse cardiac events in a subgroup of the 4.0 mm stent was not significantly higher in the BMS group than in the DES group.21 In addition, the BeStent 2 study showed that there were no significant differences in angiographic and clinical restenosis between sirolimus-eluting DES and thin strut BMS in cases with a vessel greater than 2.8 mm in diameter.22 In the BASKET trial, the benefits of DESs in reducing the rate of target vessel revascularization persisted up to 3 years in patients with stents smaller than 3 mm; however, this benefit was small and insignificant in patients with stents larger than 3 mm. Meanwhile, the 3-year MACE rate was significantly higher in patients with large coronary arteries treated with DES due to the increases in rate of late ST.23 This finding implies that DESs in large coronary arteries are more prone to late ST and may show worse long-term clinical outcome compared to BMSs. In addition, DESs are more expensive than BMS and require a longer duration of dual-antiplatelet therapy, which further increases cost.
DESs are now preferred in most PCI procedures because they reduce the incidence of restenosis and TLR. Most recent trials suggested that there were benefits to DESs relative to BMSs even in patients with acute MI.12-17 While DESs were obviously effective in reducing restenosis rates by inhibiting neointimal growth, data from a number of studies had shown conflicting results about their efficacy in the treatment of large coronary arteries.18-23 The TAXUS-IV analysis reported that there were no statistically significant benefits to DES in reducing the restenosis rate in patients with coronary arteries larger than 3.0 mm.20 The TAXUS-V study also showed that the frequency of 9-month adverse cardiac events in a subgroup of the 4.0 mm stent was not significantly higher in the BMS group than in the DES group.21 In addition, the BeStent 2 study showed that there were no significant differences in angiographic and clinical restenosis between sirolimus-eluting DES and thin strut BMS in cases with a vessel greater than 2.8 mm in diameter.22 In the BASKET trial, the benefits of DESs in reducing the rate of target vessel revascularization persisted up to 3 years in patients with stents smaller than 3 mm; however, this benefit was small and insignificant in patients with stents larger than 3 mm. Meanwhile, the 3-year MACE rate was significantly higher in patients with large coronary arteries treated with DES due to the increases in rate of late ST.23 This finding implies that DESs in large coronary arteries are more prone to late ST and may show worse long-term clinical outcome compared to BMSs. In addition, DESs are more expensive than BMS and require a longer duration of dual-antiplatelet therapy, which further increases cost.
In this study, the PRO-Kinetic stent with thin cobalt chromium strut and silicon carbide coating proved safety, efficacy, and acceptable restenosis rates in STEMI patients, especially with relatively large coronary arteries (>3.0 mm). Previous studies using PRO-Kinetic stents also showed favorable results.24-26 The low TLR and MACE rates in large vessels are at least partly related to the stent material, design, and silicon carbide coating. A thinner strut composite made with cobalt chromium alloy and the helical design gives the stent a low profile and high flexibility and helps to prevent angiographic and clinical restenosis.27,28 Amorphous silicon-carbide coated stent surfaces markedly lower the rate of platelet adhesion, platelet/fibrin activation, and leukocyte adhesion. Thus, the passive coating of PRO-Kinetic stent with silicon carbide may improve biocompatibility of stent platforms and reduce thrombogenecity.29 Based on these results, it is believed that the PRO-Kinetic stent can be considered as one of the treatment options in STEMI patients with large reference vessel diameter and relatively short lesion length. However, it seems not to be the case in the smaller vessels (≤3.0 mm), in which the binary restenosis rate was more than 50%. Although there were only 17 patients in the small vessel group and the restenosis rate in this group should be validated in a larger study, we admit that DESs are still preferred in conditions at high risk for restenosis, such as small vessels, diffuse long lesions, or diabetes.
Study limitations. First, despite the prospective and multicenter design, this study analyzed a small number of patients and the follow-up period was relatively short. Second, the rate of angiographic follow-up was lower than expected and was performed on only two-thirds of the enrolled patients. The relatively low angiographic follow-up rate may have influenced the result, especially for the secondary endpoint, late loss. In addition, there could be additional restenosis without symptom, which could influence the rate of TLR. Nowadays, however, it is recommended to assess clinically driven TLR within a time interval that precedes any protocol-mandated repeat catheterization,10 and the low rate of follow-up angiography in this study is expected to have little influence on the primary endpoint, the rate of MACE at the 6-month follow-up. Third, because we included STEMI patients with relatively large coronary arteries and short lesion length in the study, there may be selection bias. Therefore, further studies with a larger sample size and longer follow-up periods are needed to confirm our results.
Conclusion
In conclusion, the use of the PRO-Kinetic stent seems to be safe and feasible in primary PCI for acute STEMI, and shows favorable clinical and angiographic outcomes in large (>3.0 mm) coronary arteries, but not in small arteries.
References
- Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937-948.
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356(10):998-1008.
- Serruys PW, Daemen J. Are drug-eluting stents associated with a higher rate of late thrombosis than bare-metal stents? Late stent thrombosis: a nuisance in both bare metal and drug-eluting stents. Circulation. 2007;115(11):1433-1439.
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126-2130.
- Tolleson TR, Newby LK, Harrington RA, et al. Frequency of stent thrombosis after acute coronary syndromes (from the SYMPHONY and 2nd SYMPHONY trials). Am J Cardiol. 2003;92(3):330-333.
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48(1):193-202.
- Rzany A, Schaldach M. Smart material silicon carbide: reduced activation of cells and proteins on a-SiC:H-coated stainless steel. Prog Biomed Res. 2000;6(3):182-194.
- Heublein B, Pethig K, Özbek C, et al. Silicon carbide coating — a new hybrid design of coronary stents. Prog Biomed Res. 1998;3(1):33-39.
- Hamm CW, Hugenholtz PG. Silicon carbide-coated stents in patients with acute coronary syndrome. Catheter Cardiovasc Interv. 2003;60(3):375-381.
- Cutlip ED, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344-2351.
- Foley DP, Escaned J, Strauss BH, et al. Quantitative coronary angiography (QCA) in interventional cardiology: clinical application of QCA measurements. Prog Cardiovasc Dis. 1994;36(5):363-384.
- Moses JW, Mehran R, Nikolsky E, et al. Outcomes with the paclitaxel-eluting stent in patients with acute coronary syndromes: analysis from the TAXUS-IV trial. J Am Coll Cardiol. 2005;45(8):1165-1171.
- Spaulding C, Henry P, Teiger E, et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med. 2006;355(11):1093-1104.
- Menichelli M, Parma A, Pucci E, et al. Randomized trial of sirolimus-eluting stent versus bare-metal stent in acute myocardial infarction (SESAMI). J Am Coll Cardiol. 2007;49(19):1924-1930.
- Kelbaek H, Thuesen L, Helqvist S, et al. Drug-eluting versus bare metal stents in patients with ST-segment elevation myocardial infarction: eight-month follow-up in the drug elution and distal protection in acute myocardial infarction (DEDICATION) trial. Circulation. 2008;118(11):1155-1162.
- Stone GW, Lansky AJ, Pocock SJ, et al. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009;360(19):1946-1959.
- Di Lorenzo E, De Luca G, Sauro R, et al. The PASEO (paclitaxel or sirolimus-eluting stent versus bare-metal stent in primary angioplasty) randomized trial. JACC Cardiovasc Interv. 2009;2(6):515-523.
- Steinberg DH, Mishra S, Javaid A, et al. Comparison of effectiveness of bare-metal stents versus drug-eluting stents in large (> or = 3.5 mm) coronary arteries. Am J Cardiol. 2007;99(5):599-602.
- Quizhpe AR, Feres F, de Ribamar Costa J, et al. Drug-eluting stents vs bare-metal stents for the treatment of large coronary vessels. Am Heart J. 2007;154(2):373-378.
- Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350(3):221-231.
- Stone GW, Ellis SG, Cannon L, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare-metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005;294(10):1215-1223.
- Pache J, Dibra A, Mehilli J, et al. Drug-eluting stents compared with thin-strut bare stents for the reduction of restenosis: a prospective, randomized trial. Eur Heart J. 2005;26(13):1262-1268.
- Pfisterer M, Brunner-La Rocca HP, Rickenbacher P, et al. Long-term benefit-risk balance of drug-eluting vs. bare-metal stents in daily practice: does stent diameter matter? Three-year follow-up of BASKET. Eur Heart J. 2009;30(1):16-24.
- Dahm JB, Willems T, Wolpers HG, et al. Clinical investigation into the observation that silicon carbide coating on cobalt chromium stents leads to early differentiating functional endothelial layer, increased safety and DES-like recurrent stenosis rates: results of the PRO-Heal Registry (PRO-Kinetic enhancing rapid in-stent endothelialisation). EuroIntervention. 2009;4(4):502-508.
- Kornowski R, Vaknin-Assa H, Ukabi S, et al. PRO-Kinetic: results from an “all-comers” single centre clinical experience. EuroIntervention. 2009;5(1):109-114.
- Berlin T, Rozenbaum E, Arbel J, et al. Six- and twelve-month clinical outcomes after implantation of prokinetic BMS in patients with acute coronary syndrome. J Interv Cardiol. 2010;23(4):377-381.
- Pache J, Kastrati A, Mehilli J, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol. 2003;41(8):1283-1288.
- Fujimoto H, Tao S, Masuda J, et al. The efficacy of bare-metal stent implantation for patients with acute myocardial infarction in the drug-eluting stent era. J Cardiol. 2008;51(3):189-195.
- Hansi C, Arab A, Rzany A, et al. Differences of platelet adhesion and thrombus activation on amorphous silicon carbide, magnesium alloy, stainless steel, and cobalt chromium stent surfaces. Catheter Cardiovasc Interv. 2009;73(4):488-496.
_________________________________________________
*Joint first authors.
From the 1Korea University Ansan Hospital, Ansan, Korea, 2Seoul National University Bundang Hospital, Seongnam, Korea, 3Seoul National University Boramae Municipal Hospital, Seoul, Korea, 4Jeju National University Hospital, Jeju, Korea, and 5Chungbuk National University Hospital, Cheongju, Korea.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Funding: The authors received grant from IMT Medical. The funding source of the study had no role in study design, data collection, monitoring, analysis, interpretation, or writing of the manuscript.
Manuscript submitted December 13, 2011, provisional acceptance given December 28, 2011, final version accepted January 25, 2012.
Address for correspondence: Tae-Jin Youn, MD, PhD, Division of Cardiology, Department of Internal Medicine, College of Medicine, Seoul National University and Cardiovascular Center, Seoul National University Bundang Hospital, 166 Gumi-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, 463-707, Republic of Korea. Email: ytjmd@snubh.org












