Effect of Changes in Contractility on Pressure Drop Coefficient and Fractional Flow Reserve in a Porcine Model
 Abstract: Objectives and Background. Decisions based on invasive functional diagnostic measurements are often made in the setting of fluctuating hemodynamic variables that may alter resting or hyperemic measurements. The purpose of this investigation is to analyze the effect of myocardial contractility (CY) on invasive functional parameters. We hypothesize that the pressure drop coefficient (CDPe; ratio of pressure drop to distal dynamic pressure) and fractional flow reserve (FFR; ratio of average pressures distal and proximal to a stenosis) are not affected by fluctuations in CY and can distinguish between different severities of epicardial stenosis. Methods. Simultaneous measurements of distal coronary-arterial pressure and velocity were performed in 10 pigs using a dual-sensor tipped guidewire for heart rate (HR) <110 bpm and HR >110 bpm, in the presence of coronary lesions of <50% area stenosis (AS) and >50% AS. Variations in myocardial function and vascular resistance were induced by atrial pacing, papaverine and balloon obstruction, respectively. The maximum rate of rise of left ventricular pressure ([dp/dt]max) was the index of contractility. The contractile function of the heart was empirically defined as CY >900 mm Hg/sec (higher) and CY <900 mm Hg/sec (normal). Results. For CY >900 mm Hg/sec, under AS <50% and AS >50%, the mean values of FFR (0.91 ± 0.02 and 0.78 ± 0.02), and CDPe (15.6 ± 5.3 and 70.7 ± 24.7) were significantly different (P<.05). Similarly, for CY <900 mm Hg/sec, under AS <50% and AS >50%, the mean values of FFR (0.83 ± 0.04 and 0.63 ± 0.04), and CDPe (43.8 ± 14.9 and 191.8 ± 61.4) were also significantly different (P<.05). Conclusions. Both FFR and CDPe could effectively distinguish between stenosis severity at normal and higher levels of myocardial contractility.
Abstract: Objectives and Background. Decisions based on invasive functional diagnostic measurements are often made in the setting of fluctuating hemodynamic variables that may alter resting or hyperemic measurements. The purpose of this investigation is to analyze the effect of myocardial contractility (CY) on invasive functional parameters. We hypothesize that the pressure drop coefficient (CDPe; ratio of pressure drop to distal dynamic pressure) and fractional flow reserve (FFR; ratio of average pressures distal and proximal to a stenosis) are not affected by fluctuations in CY and can distinguish between different severities of epicardial stenosis. Methods. Simultaneous measurements of distal coronary-arterial pressure and velocity were performed in 10 pigs using a dual-sensor tipped guidewire for heart rate (HR) <110 bpm and HR >110 bpm, in the presence of coronary lesions of <50% area stenosis (AS) and >50% AS. Variations in myocardial function and vascular resistance were induced by atrial pacing, papaverine and balloon obstruction, respectively. The maximum rate of rise of left ventricular pressure ([dp/dt]max) was the index of contractility. The contractile function of the heart was empirically defined as CY >900 mm Hg/sec (higher) and CY <900 mm Hg/sec (normal). Results. For CY >900 mm Hg/sec, under AS <50% and AS >50%, the mean values of FFR (0.91 ± 0.02 and 0.78 ± 0.02), and CDPe (15.6 ± 5.3 and 70.7 ± 24.7) were significantly different (P<.05). Similarly, for CY <900 mm Hg/sec, under AS <50% and AS >50%, the mean values of FFR (0.83 ± 0.04 and 0.63 ± 0.04), and CDPe (43.8 ± 14.9 and 191.8 ± 61.4) were also significantly different (P<.05). Conclusions. Both FFR and CDPe could effectively distinguish between stenosis severity at normal and higher levels of myocardial contractility.
J INVASIVE CARDIOL 2012;24(1):6-12
Key words: coronary disease, contractility, stenosis, cardiac hemodynamics, catheterization
_______________________________________
Coronary angiography is the current standard for detecting epicardial coronary artery disease. However, measuring coronary hemodynamic parameters (pressure and velocity) provides unique physiologic information that complements anatomic (angiographic) evaluation and facilitates appropriate decision-making for treatment in the catheterization laboratory. Several approaches have been proposed in cardiac catheterization laboratories alongside coronary angiography to better define the functional significance of epicardial coronary stenosis. These methods include the evaluation of coronary flow reserve (CFR)1 and fractional flow reserve (FFR).2,3 Moreover, with the introduction of technologically improved Doppler-tipped flow wires4 and pressure wires,5 the functional assessment of coronary disease can now be performed along with coronary angiography.
A limitation for the use of invasive coronary diagnostic parameters is that fluctuations in hemodynamic factors such as heart rate,6 blood pressure,7 and myocardial contractility8-13 may alter resting or hyperemic flow and can cause ambiguity in the interpretation of the results.13,14 Hence, the evaluation of coronary circulation should rely on methodologies independent of these hemodynamic changes. Previous human studies with FFR have shown that it is independent of variations in heart rate (HR) and contractility (CY).8,15
FFR is an established invasive clinical parameter used for assessing physiological significance of epicardial disease. FFR is a non-dimensional ratio, which has a lower bound of ‘0’ representing complete vessel obstruction and upper bound of ‘1’ representing no obstruction and normal flow. Based on extensive clinical trials, a cutoff value of 0.75-0.8 for FFR was shown to indicate hemodynamic significance of coronary stenosis.15-18 Some limitations of FFR include assumption of zero central venous pressure and constant minimal myocardial resistance at maximal hyperemia. There are also some controversial data suggesting that FFR may be affected by the distal microvascular resistance and the inability to achieve maximal hyperemia.19
To overcome these issues, simultaneous measurement of pressure drop and velocity is proposed for the diagnosis of epicardial coronary stenosis. Recently, we introduced the functional index, the pressure drop coefficient20 (CDPe; the ratio of trans-stenotic pressure drop [∆p] to distal dynamic pressure, [½ × blood density (ρ) × APV2; where APV equals the average peak flow velocity] measured under maximal hyperemia). This parameter is a non-dimensional ratio, derived from fundamental fluid dynamic principles. The functional measurements (∆p and APV) necessary for the evaluation of CDPe can be readily obtained simultaneously during routine cardiac catheterization procedures using a dual-sensor tipped guidewire. The CDPe was validated in in vitro20,21 and in vivo animal studies.22-24 The cutoff values for CDPe similar to FFR are currently being investigated under clinical settings.
CDPe was also shown to be independent of heart rate in our recent in vivo animal study.23 Consequently, we report herein the effect of variable levels of myocardial contractility on FFR and CDPe in an animal model based on two (high and low) functional thresholds (area blockage).
Methods
Animal model. Traditionally, swine (or pig) and canine (or dog) have been used for development, and validation of several hemodynamic diagnostic parameters for evaluating coronary stenosis severity. The coronary circulation and coronary artery anatomy of canine heart differs significantly from that of healthy human heart,25ie, canines have a variable pre-existing coronary collateral network, whereas the native coronary circulation of the porcine heart is remarkably similar to that of a human devoid of any epicardial stenoses.26-28 It was also reported that, under several conditions of stress, the responses of pigs, in contrast to dogs, are similar to those of humans.29-31 It should be noted that the porcine model also permits us to collect data in a coronary circulation that has no pre-existing collateral network under conditions of transiently applied coronary artery stenosis. Thus in this study, the porcine model was used to make baseline observations and conclusions, giving a more controlled data set that could be extended in the future to assess the effect with and without collateral flow in humans.
Animal preparation. The animal study protocol was approved by the Institutional Animal Care and Use Committee at the University of Cincinnati and Cincinnati Children’s Hospital Medical Center. The in vivo study was performed on 10 Yorkshire pigs (42 ± 3 kg). The animals were fasted for 24 hours before the procedure and were premedicated with intramuscular ketamine (20 mg/kg) or telazol (2-7 mg/kg), as well as atropine (0.4 mg/kg), xylazine (2 mg/kg), and buprenorphine (0.005 mg/kg). General anesthesia was maintained with 2% of Isoflurane and endotracheal oxygen supply as per the surgical procedural standards.32 Heart rate, oxygen saturation and end-tidal CO2 levels were monitored every 15 minutes and ventilator changes were made as needed to maintain these values in the normal range.
Catheterization and angiography. In the closed-chest pig heart model, an arterial sheath was placed by surgical cut-down in the right carotid, femoral arteries, and right jugular vein. This procedure was followed by the insertion of a 7 Fr guide catheter via femoral artery that was advanced under fluoroscopic guidance to the left main coronary ostium. An intravenous dose of heparin (300 U/kg) was injected immediately. Angiographic images were used to select a segment of the left anterior descending (LAD) artery with no significant sidebranches.24 A pacing wire was placed into the right atrium through the right jugular vein to pace the heart using an external pulse generator (5330 PSA; Medtronic, Inc.). A 5 Fr Mikro-Tip catheter (Millar Instruments) was then introduced into the left ventricle via the right carotid artery to obtain HR and left ventricular pressure (LVP) measurements.
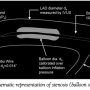 Anatomical measurements. After engaging the guide catheter at the coronary ostium, the native vessel lumen area was measured by motorized pullback (1 mm/sec) of the intravascular ultrasound (IVUS) catheter (2 Fr In-Vision Gold; Volcano Therapeutics). Before the IVUS measurements, a bolus dose (0.1-1.0 µg/kg) of intracoronary nitroglycerin was injected to eliminate spasms that might be induced due to insertion of the IVUS catheter. Based on the IVUS and angiographic images, a portion of the LAD was selected for creating epicardial flow obstruction, induced by inflating a coronary angioplasty balloon (Voyager; Guidant Corporation). The choice of the balloon diameter was determined based on IVUS images and data table provided by the manufacturer to produce predictable coronary obstruction.
Anatomical measurements. After engaging the guide catheter at the coronary ostium, the native vessel lumen area was measured by motorized pullback (1 mm/sec) of the intravascular ultrasound (IVUS) catheter (2 Fr In-Vision Gold; Volcano Therapeutics). Before the IVUS measurements, a bolus dose (0.1-1.0 µg/kg) of intracoronary nitroglycerin was injected to eliminate spasms that might be induced due to insertion of the IVUS catheter. Based on the IVUS and angiographic images, a portion of the LAD was selected for creating epicardial flow obstruction, induced by inflating a coronary angioplasty balloon (Voyager; Guidant Corporation). The choice of the balloon diameter was determined based on IVUS images and data table provided by the manufacturer to produce predictable coronary obstruction.
The balloon was mounted on the dual-sensor tipped guidewire used for pressure and velocity measurements. The configuration of sensor-tipped guidewire and the balloon placement used to calculate the % area stenosis (AS) are shown in Figure 1. The procedure of inflating a balloon to different pressures to create intraluminal obstructions of varying severity (<50% to >50% by area) is similar to our previous studies22-24 and the study by MacCarthy et al.33 Linear variation of diameter with change in inflation pressure, as per the manufacturer’s data sheet (Voyager balloons; Guidant, Inc.) for an individual balloon, was used to calculate the percentage area intraluminal obstructions.
Functional measurements. In all 10 pigs, the phasic distal coronary pressure (pd) and APV were measured simultaneously by a dual-sensor tipped guidewire (Combowire; Volcano Therapeutics), as shown in Figure 1. The mean proximal aortic pressure (pa) was continuously recorded from the guide catheter. HR and LVP were continuously recorded using the Millar catheter connected to a 4-channel transducer amplifier (Sonometric Systems). LVP signals were analyzed on the CardioSoft data acquisition and analysis system (Sonometric Corporation). CY was evaluated from the maximum rate of rise of LVP ([dp/dt]max) as a surrogate indicator of myocardial contractility.34-37 The hemodynamic measurements were performed at baseline flow and at maximal hyperemic flow (obtained by injecting 10 mg of intracoronary papaverine). It should be noted that only mechanical pacing was performed for varying the contractile function of the heart. Pacing of the heart using an inotropic intervention (eg, beta adrenergic stimulation) was avoided in order to eliminate unknown interactions between such a drug and papaverine, which in turn could have an influence on the hyperemic condition of the coronary circulation. Values of pd, pa, APV, and FFR were recorded in the Combo Map system (Volcano Therapeutics Inc.) for various HR conditions, viz. HR <110 bpm and HR >110 bpm, using atrial pacing. Measurements were recorded only after maximal hyperemia was achieved. Typically, we waited 30 seconds after papaverine injection for 3 consecutive sets of similar readings, for a specific stenotic condition.
After obtaining the first hyperemic readings (for a given HR and AS) as described above, we had the pig rest for about 3-5 minutes (with the balloon deflated), until the parameter values of HR, pressure, and velocity returned to normal. We changed the HR only after the parameters returned to normal and then injected papaverine via intracoronary route to induce hyperemia for the next balloon inflation and data acquisition. AS was varied from 5% to 90% through balloon obstruction and for each level of AS, HR was varied from 90 bpm to 190 bpm.
Calculation of FFR. FFR is defined as the ratio of maximal myocardial blood flow distal to a stenotic artery (Q) to the theoretical maximal flow in the absence of the stenosis (QN). Clinically, maximal flow is achieved by administering intracoronary papaverine or intracoronary adenosine. Under this maximum flow (hyperemia), the resistance (R) imposed by the myocardial bed is minimal and blood flow is proportional to driving pressure. FFR can thus be expressed as:
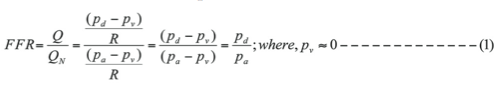
where pa, pd, and pv represent the mean aortic, distal coronary, and central venous pressures obtained at hyperemia. Generally, central venous pressure is close to zero for normal microvasculature.
Calculation of CDPe. CDPe has been previously described in detail in the literature.20,22,24,38 Briefly, CDPe is defined as the ratio of trans-stenotic pressure drop [∆p = pa - pd] to distal dynamic pressure. Distal dynamic pressure is calculated as the product of blood density (ρ), the square of average peak flow velocity (APV) and a constant value of 0.5; (0.5 × ρ × APV2), measured at hyperemia. Blood density, ρ, does not change significantly at hyperemia and thus can be assumed to have a constant value (1.05 gm/cm3).22,38

where, pa and pd are average pressures measured proximal and distal to the stenosis at hyperemia, respectively.
Statistical analysis. Analysis using one-way repeated measure ANOVA was performed based on logarithm-transformed data values, of which the distribution did not significantly deviate from normality (Kolmogrov-Smirnov statistics with Lilliefor’s significance level, P>.2). A compound symmetry (CS) correlation structure was assumed between repeated measurements. Data analysis was performed on SAS 9.1.3 (SAS Institute Inc.) with P<.05 used as the probability level to accept statistical significance. All functional measurements and hemodynamic parameters are represented as mean ± SE.
Stratification of hemodynamic conditions. Studies were performed at two different thresholds, HR and % epicardial coronary area stenosis (AS). Data for this study were analyzed according to myocardial contractility and AS. Similar to previous studies by Banerjee et al22 and Sinha Roy et al,24 mild stenosis was classified as AS <50% and intermediate stenosis as AS >50% for the analysis. Based on the data values obtained, the group-mean values of CY for AS <50% (1300 mm Hg/sec) and AS >50% (750 mm Hg/sec) were determined. Further, in order to study the effect of CY on invasive diagnostic parameters, we empirically chose CY to be a binary variable separated at 1000 mm Hg/sec and 900 mm Hg/sec, which falls around the overall mean value of CY between AS <50% and AS >50%. However, the data distribution was found to be better and balanced for 900 mm Hg/sec when compared to that of 1000 mm Hg/sec. Hence, we chose the cutoff value of 900 mm Hg/sec which was used to define normal (CY <900 mm Hg/sec) and high (CY >900 mm Hg/sec) myocardial contractility.
Results
 A total of 121 simultaneous pressure-velocity readings were recorded in 10 pigs under different hemodynamic conditions. The group with CY >900 mm Hg/sec has 53 readings for AS <50%, 18 readings for AS >50%. The group with CY <900 mm Hg/sec has 18 readings for AS <50%, 32 readings for AS >50%. The mean %AS varied from 0.27 ± 0.02 to 0.65 ± 0.01 for lesions of AS <50% and AS >50%, respectively. The FFR and CDPe were calculated from the above pressure-velocity data.
A total of 121 simultaneous pressure-velocity readings were recorded in 10 pigs under different hemodynamic conditions. The group with CY >900 mm Hg/sec has 53 readings for AS <50%, 18 readings for AS >50%. The group with CY <900 mm Hg/sec has 18 readings for AS <50%, 32 readings for AS >50%. The mean %AS varied from 0.27 ± 0.02 to 0.65 ± 0.01 for lesions of AS <50% and AS >50%, respectively. The FFR and CDPe were calculated from the above pressure-velocity data.
 Relation between maximum rate of rise of LVP and HR. To investigate the relation between CY (using ([dp/dt]max) as surrogate) with increasing HR, a regression line was fitted between the two for the different AS groups (AS <50% and AS >50%). In the present closed-chest pig model, the atrium was paced at varying rates and a direct linear correlation was found between CY and HR as shown in Figures 2 and 3. Figures 2A and 2B refer to the linear regression plot for HR vs CY under AS <50%, showing an increase in CY for increasing HR. This correlates to a normal physiological response. Figures 3A and 3B refer to the linear regression plot for HR vs CY where AS >50%. From Figure 3A and Figure 3B, for AS >50%, we observe that CY decreases with increasing HR. This is an expected observation, since an increase in HR in the presence of a significant coronary stenosis and limiting flow leads to an increase in oxygen demand and will result in a decrease in CY.
Relation between maximum rate of rise of LVP and HR. To investigate the relation between CY (using ([dp/dt]max) as surrogate) with increasing HR, a regression line was fitted between the two for the different AS groups (AS <50% and AS >50%). In the present closed-chest pig model, the atrium was paced at varying rates and a direct linear correlation was found between CY and HR as shown in Figures 2 and 3. Figures 2A and 2B refer to the linear regression plot for HR vs CY under AS <50%, showing an increase in CY for increasing HR. This correlates to a normal physiological response. Figures 3A and 3B refer to the linear regression plot for HR vs CY where AS >50%. From Figure 3A and Figure 3B, for AS >50%, we observe that CY decreases with increasing HR. This is an expected observation, since an increase in HR in the presence of a significant coronary stenosis and limiting flow leads to an increase in oxygen demand and will result in a decrease in CY.
 Diagnostic parameters for normal contractile conditions. To investigate the effect of normal myocardial contractility (CY), FFR and CDPe were evaluated for AS <50% and AS >50%. Figures 4A and 4B refer to the main effects of %AS for diagnostic parameters under normal contractility conditions, ie, CY <900 mm Hg/sec. Figure 4A shows the bar graph of FFR as a function of AS. The mean values of FFR for AS <50% (0.83 ± 0.04) and AS >50% (0.63 ± 0.04) are significantly different (P<.05). Figure 4B shows the bar graph of CDPe as a function of AS. The mean values of CDPe for AS <50% (43.8 ± 14.9) and AS >50% (191.8 ± 61.4) are significantly different (P<.05). Thus, the diagnostic parameters (FFR and CDPe) can differentiate between stenosis severities under normal contractile condition of the heart.
Diagnostic parameters for normal contractile conditions. To investigate the effect of normal myocardial contractility (CY), FFR and CDPe were evaluated for AS <50% and AS >50%. Figures 4A and 4B refer to the main effects of %AS for diagnostic parameters under normal contractility conditions, ie, CY <900 mm Hg/sec. Figure 4A shows the bar graph of FFR as a function of AS. The mean values of FFR for AS <50% (0.83 ± 0.04) and AS >50% (0.63 ± 0.04) are significantly different (P<.05). Figure 4B shows the bar graph of CDPe as a function of AS. The mean values of CDPe for AS <50% (43.8 ± 14.9) and AS >50% (191.8 ± 61.4) are significantly different (P<.05). Thus, the diagnostic parameters (FFR and CDPe) can differentiate between stenosis severities under normal contractile condition of the heart.
 Diagnostic parameters for high contractile conditions. To investigate the effect of higher myocardial contractility (CY >900 mm Hg/sec) on diagnostic parameters, FFR and CDPe are evaluated for AS <50% and AS >50%. Figure 5 shows the main effects of %AS for diagnostic parameters under higher contractility conditions, ie, CY >900 mm Hg/sec. Figure 5A shows the bar graph of FFR as a function of AS. The mean values of FFR for AS <50% (0.91 ± 0.02) and AS >50% (0.78 ± 0.02) are significantly different (P<.05). Figure 5B shows the bar graph of CDPe as a function of AS. The mean values of CDPe for AS <50% (15.6 ± 5.3) and AS >50% (70.7 ± 24.7) are significantly different (P<.05). Thus, the diagnostic parameters FFR and CDPe can differentiate between stenosis severities under high myocardial contractility conditions.
Diagnostic parameters for high contractile conditions. To investigate the effect of higher myocardial contractility (CY >900 mm Hg/sec) on diagnostic parameters, FFR and CDPe are evaluated for AS <50% and AS >50%. Figure 5 shows the main effects of %AS for diagnostic parameters under higher contractility conditions, ie, CY >900 mm Hg/sec. Figure 5A shows the bar graph of FFR as a function of AS. The mean values of FFR for AS <50% (0.91 ± 0.02) and AS >50% (0.78 ± 0.02) are significantly different (P<.05). Figure 5B shows the bar graph of CDPe as a function of AS. The mean values of CDPe for AS <50% (15.6 ± 5.3) and AS >50% (70.7 ± 24.7) are significantly different (P<.05). Thus, the diagnostic parameters FFR and CDPe can differentiate between stenosis severities under high myocardial contractility conditions.
Discussion
Invasive assessment of coronary flow can provide clinically useful information in patients with known or suspected ischemic heart disease. It has been emphasized that microvascular disease and hemodynamic variables (HR, AS, blood pressure, and CY) can alter resting or maximal flow conditions.12,13 With improvements in the guidewire technology, assessment of variability of these factors has been made easier. In the present in vivo animal study, we further validated CDPe20,22,24 in relation to FFR under different conditions of myocardial CY. Measurements were made for various degrees of epicardial stenosis (AS <50% and AS >50%) and HR variation (HR <110 bpm and HR >110 bpm). The maximum rate of rise of LVP ([dp/dt]max) was used as an index of CY. The effect of variable levels of myocardial CY on FFR and CDPe in an animal model for different anatomic thresholds (area blockage) was assessed. The major findings from the study are: (1) CDPe is easy to calculate, and can distinguish between different levels of AS under normal and high contractile conditions; and (2) FFR can distinguish between different degrees of coronary stenosis for normal and high contractile conditions.
FFR represents the ratio of maximal flow through a stenosed vessel with that of maximal flow achieved if the vessel was disease free. FFR has been proposed and validated as being more specific to epicardial obstructions and independent of changes in external hemodynamic conditions.3,8 However, in a theoretical analysis, it was demonstrated that an increase in aortic pressure (consequential increase in flow) will lead to a proportionately smaller increase in distal pressure,39 owing to the non-linear nature of pressure-drop flow relationship across a stenosis,40,41 which may influence FFR measurements. Furthermore, FFR presumes maximal hyperemia in the vascular bed being interrogated, which is not reliably achieved in certain patients.42 The effect of microvascular dysfunction on FFR measurements remains an area of controversy. In the presence of microvascular dysfunction, the maximal hyperemic flow may not be fully achieved and thus may indicate falsely higher FFR values19,43 (underestimating severity of stenosis). Further, in an animal model, Banerjee et al22 have shown that FFR could not differentiate between normal and abnormal microcirculation for different degrees of stenosis.
To overcome these issues, simultaneous measurements of pressure and velocity (ideally with a dual-sensor guidewire) have been proposed. These simultaneous measurements can potentially eliminate ambiguity in diagnostic evaluation of coronary stenosis severity before and after angioplasty or stenting.
Notably, Smalling et al44 have shown that myocardial function remained unaffected despite a low perfusion pressure, suggesting that regional contractile function depends on blood flow rather than perfusion pressure, despite the fact that these two factors are interdependent. Blood flow (Q) through a stenosis causes a translesional pressure-drop (Δp), which can be expressed as the sum of frictional losses along entrance and tightest segment (throat) of the stenosis, and inertial losses at the downstream end of stenosis. This can be described by: Δp = AvQ + BmQ2, where Av and Bm are the viscous (friction) and momentum-change (area change) loss coefficients, respectively.40,41 These coefficients depend on the properties of blood and on detailed geometry of stenosis. Stenosis resistance is defined as the ratio of translesional pressure-drop to coronary flow (Δp/Q), and is related to flow as Rs = Av + BmQ. Thus, the use of both pressure and velocity measurements in evaluation of coronary stenosis has been shown to be more accurate.22,45 Owing to the non-linear nature of pressure drop-flow relationship, it is rather appropriate that the prediction of functional severity of a stenosis should be based on these two interdependent variables. Banerjee et al22,38 have recently proposed the pressure drop coefficient (CDPe), a non-dimensional parameter which is a measure of stenosis severity as it combines hyperemic pressure drop and velocity measurements.
Physiologically, the extent of reduction in maximal hyperemic flow due to microvascular dysfunction is higher than that due to epicardial stenosis.22 The square of maximal hyperemic flow in the denominator of CDPe magnifies this reduction, thus providing an increased resolving power for CDPe to more accurately evaluate the status of epicardial vessel and microcirculation simultaneously. The values of CDPe range from zero to infinity.22 Also, from its definition, CDPe can account for both the momentum change and viscous-related pressure losses across a stenosis as described by Gould40 and Young.41 In addition, in our recent study,23 CDPe was found to be independent of variation in HR, similar to FFR. Moreover, in the presence of microvascular dysfunction and submaximal hyperemia, pressure drop and blood flow are affected in the same direction. Hence, the trend of CDPe is expected to be similar under such conditions and thus, it can possibly be used to evaluate stenosis severity under baseline condition (without needing a hyperemia).
Relation between HR and contractility under different conditions of epicardial stenosis. An interesting observation during this study is the relationship between HR and CY. Under nonischemia producing mild coronary stenosis, we noticed an increase in CY (as measured by rate of rise of LVP) as HR is increased (Figures 2A and 2B). This correlates to a normal physiological response. In contrast, with significant epicardial coronary stenosis, a decline in myocardial CY is noticed with increasing HR (Figures 3A and 3B). This inverse relationship can be readily explained by the fact that under ischemic conditions and increasing HR, mismatch between oxygen demands and supply will result in myocardial ischemia and a decline in CY.
Limitations
Anesthesia. The pathophysiological responses to flow obstruction and also the response of pharmacological agents may be influenced by the presence of anesthetic agents.46-50 In this study, we have used 2% isoflurane as an anesthetic agent. Hickey et al51 have reported that 2% isoflurane results in an increase in coronary blood flow and reduction in coronary vascular resistance. Further, anesthesia can also affect the control of arterial pressure, cardiac output, and HR.46,52 These changes can affect the myocardial CY values, since CY is measured using an indirect index (LV [dp/dt]max), which itself depends on HR and cardiac afterload.35
Hemodynamic conditions. The infusion of cardiac medication (papaverine) can also induce an increase in HR and contractility,8,53 and thus, these hemodynamic variables are interdependent. The study protocol was designed to mimic the alterations in these hemodynamic variables during regular interventional procedures, during which these variables are closely interrelated and do not fluctuate independently. In our analysis, we used the actual rate of rise of LVP ([dp/dt]max) to quantify and correlate with myocardial contractility.
Flow measurements. The epicardial arterial obstruction was induced internally by inflating the angioplasty balloon. Errors in flow measurement could occur if downstream placement of the Doppler flow sensor relative to the angioplasty balloon24 was inaccurate. While placing the sensor downstream to the balloon, arterial branches were avoided between the sensor and the balloon. At the same time, sufficient distance between the two was maintained in order to avoid downstream instabilities in flow measurement.
Balloon obstruction vs arterial plaque. The internal balloon occlusion represents different hemodynamic conditions compared to the plaque stenosis20,22,24 in terms of spatial velocity profiles, eccentricity effect, additional flow resistance (offered by the balloon shaft, diameter = 0.79 mm; Figure 1) and increased viscous losses. This could result in a difference in the overall magnitude of CDPe calculated for internal balloon obstruction and arterial plaque stenosis. However, it is expected that this parameter would follow a similar trend if the resistance offered by the internal balloon obstruction and the balloon shaft are lumped together and compared with external stenotic resistance.
Collateral flow. In humans, the effect of collateral flow might play an important role in the reperfusion of the vascular bed that is originally perfused by the stenosed artery. However, pig hearts are not known to have significant coronary collaterals. Hence, the effect of collateral flow could not be studied in this porcine model study.
Conclusion
In this study, simultaneous measurements of distal coronary-arterial pressure and flow were performed using a dual-sensor tipped guidewire for low HR and high HR, in the presence of epicardial coronary lesions of <50% AS and >50% AS. The mean values of FFR and CDPe for <50% AS and >50% AS were significantly different. We conclude therefore that CDPe and FFR can detect the severity of AS under both normal and high contractile conditions of the heart.
Acknowledgments. The authors are also grateful to Dr. Mahesh Krishnamoorthy, Dr. Subhashish Das Gupta of Fluid Heat and Mass transfer in Biological systems and MEMS devices laboratory at University of Cincinnati for their assistance during the experiments. The authors deeply regret the sudden and untimely death of contributing author Dr. W. M. Gottliebson. We’ll not only miss his collaboration and understanding of engineering in medicine, but more than that, his friendship.
References
- Gould KL, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol. 1974;33(2):87-94.
- Pijls NH, van Schaardenburgh P, Manoharan G, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the defer study. J Am Coll Cardiol. 2007;49(21):2105-2111.
- Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87(4):1354-1367.
- Doucette JW, Corl PD, Payne HM, et al. Validation of a doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85(5):1899-1911.
- Emanuelsson H, Dohnal M, Lamm C, Tenerz L. Initial experiences with a miniaturized pressure transducer during coronary angioplasty. Cathet Cardiovasc Diagn. 1991;24(2):137-143.
- Domenech RJ, Goich J. Effect of heart rate on regional coronary blood flow. Cardiovasc Res. 1976;10(2):224-231.
- Ohtsuka S, Kakihana M, Sugishita Y, Ito I. Effects of the rise in aortic pressure on coronary flow reserve in dogs. Comparison between constriction of the descending thoracic aorta and injection of methoxamine. Jpn Heart J. 1987;28(3):403-412.
- de Bruyne B, Bartunek J, Sys SU, Pijls NH, Heyndrickx GR, Wijns W. Simultaneous coronary pressure and flow velocity measurements in humans. Feasibility, reproducibility, and hemodynamic dependence of coronary flow velocity reserve, hyperemic flow versus pressure slope index, and fractional flow reserve. Circulation. 1996;94(8):1842-1849.
- Iwanaga S, Ewing SG, Husseini WK, Hoffman JI. Changes in contractility and afterload have only slight effects on subendocardial systolic flow impediment. Am J Physiol. 1995;269(4 Part 2):H1202-H1212.
- Marzilli M, Goldstein S, Sabbah HN, Lee T, Stein PD. Modulating effect of regional myocardial performance on local myocardial perfusion in the dog. Circ Res. 1979;45(5):634-641.
- Rossen JD, Winniford MD. Effect of increases in heart rate and arterial pressure on coronary flow reserve in humans. J Am Coll Cardiol. 1993;21(2):343-348.
- Hoffman JI. A critical view of coronary reserve. Circulation. 1987;75(1 Pt 2):I6-I21.
- Klocke FJ. Measurements of coronary flow reserve: defining pathophysiology versus making decisions about patient care. Circulation. 1987;76(6):1183-1189.
- Hoffman JI. Maximal coronary flow and the concept of coronary vascular reserve. Circulation. 1984;70(2):153-159.
- Pijls NH, Van Gelder B, Van der Voort P, et al. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995;92(11):3183-3193.
- Fearon WF, Tonino PAL, De Bruyne B, Siebert U, Pijls NHJ. Rationale and design of the fractional flow reserve versus angiography for multivessel evaluation (FAME) study. Am Heart J. 2007;154(6):632-636.
- Lederman SJ, Menegus MA, Greenberg MA. Fractional flow reserve. ACC Current Journal Review. 1997;6:34-35.
- Silber S, Albertsson P, Avilés FF, et al. Guidelines for percutaneous coronary interventions. Eur Heart J. 2005;26(8):804-847.
- Pijls NH, Kern MJ, Yock PG, De Bruyne B. Practice and potential pitfalls of coronary pressure measurement. Catheter Cardiovasc Interv. 2000;49(1):1-16.
- Banerjee RK, Ashtekar KD, Helmy TA, Effat MA, Back LH, Khoury SF. Hemodynamic diagnostics of epicardial coronary stenoses: in vitro experimental and computational study. Biomed Eng Online. 2008;7:24.
- Peelukhana SV, Back LH, Banerjee RK. Influence of coronary collateral flow on coronary diagnostic parameters: an in vitro study. J Biomech. 2009;42(16):2753-2759.
- Banerjee RK, Ashtekar KD, Effat MA, et al. Concurrent assessment of epicardial coronary artery stenosis and microvascular dysfunction using diagnostic endpoints derived from fundamental fluid dynamics principles. J Invasive Cardiol. 2009;21(10):511-517.
- Kolli KK, Banerjee RK, Peelukhana SV, et al. Influence of heart rate on fractional flow reserve, pressure drop coefficient, and lesion flow coefficient for epicardial coronary stenosis in a porcine model. Am J Physiol Heart Circ Physiol. 2011;300(1):H382-H387.
- Sinha Roy A, Back MR, Khoury SF, et al. Functional and anatomical diagnosis of coronary artery stenoses. J Surg Res. 2008;150(1):24-33.
- Gould K. Coronary artery stenosis and reversing atherosclerosis. New York: Chapman & Hall; 1997.
- Bloor CM, White FC. Functional development of the coronary collateral circulation during coronary artery occlusion in the conscious dog. Am J Pathol. 1972;67(3):483-500.
- Millard RW. Induction of functional coronary collaterals in the swine heart. Basic Res Cardiol. 1981;76(5):468-473.
- Mills I, Fallon JT, Wrenn D, et al. Adaptive responses of coronary circulation and myocardium to chronic reduction in perfusion pressure and flow. Am J Physiol. 1994;266(2 Pt 2):H447-H457.
- Sanders M, White F, Bloor C. Cardiovascular responses of dogs and pigs exposed to similar physiological stress. Comparative Biochemistry and Physiology Part A: Physiology. 1977;58:365-370.
- Swindle MM. Swine as replacements for dogs in the surgical teaching and research laboratory. Lab Anim Sci. 1984;34(4):383-385.
- Tumbleson ME. Swine in biomedical research. V. 2. 1986.
- Aarnoudse W, Fearon WF, Manoharan G, et al. Epicardial stenosis severity does not affect minimal microcirculatory resistance. Circulation. 2004;110(15):2137-2142.
- MacCarthy P, Berger A, Manoharan G, et al. Pressure-derived measurement of coronary flow reserve. J Am Coll Cardiol. 2005;45(2):216-220.
- Drake-Holland AJ, Mills CJ, Noble MI, Pugh S. Responses to changes in filling and contractility of indices of human left ventricular mechanical performance. J Physiol. 1990;422:29-39.
- Mason DT. Usefulness and limitations of the rate of rise of intraventricular pressure (dp-dt) in the evaluation of myocardial contractility in man. Am J Cardiol. 1969;23(4):516-527.
- Richmond DR, Angus JA, Goodman AH, Cobbin LB. The effect of heart rate on indices of myocardial contractility in the dog. Clin Exp Pharmacol Physiol. 1975;2(6):469-479.
- Van den Bos GC, Elzinga C, Westerhof N, Noble MI. Problems in the use of indices of myocardial contractility. Cardiovasc Res. 1973;7(6):834-848.
- Banerjee RK, Sinha Roy A, Back LH, Back MR, Khoury SF, Millard RW. Characterizing momentum change and viscous loss of a hemodynamic endpoint in assessment of coronary lesions. J Biomech. 2007;40(3):652-662.
- Siebes M, Chamuleau SA, Meuwissen M, Piek JJ, Spaan JA. Influence of hemodynamic conditions on fractional flow reserve: parametric analysis of underlying model. Am J Physiol Heart Circ Physiol. 2002;283(4):H1462-H1470.
- Gould KL. Pressure-flow characteristics of coronary stenoses in unsedated dogs at rest and during coronary vasodilation. Circ Res. 1978;43(2):242-253.
- Young DF. Fluid mechanics of arterial stenosis. ASME J Biochem Eng. 1979;101:157-175.
- Kern MJ, Samady H. Current concepts of integrated coronary physiology in the catheterization laboratory. J Am Coll Cardiol. 2010;55(3):173-185.
- Hoffman JI. Problems of coronary flow reserve. Ann Biomed Eng. 2000;28(8):884-896.
- Smalling RW, Kelley K, Kirkeeide RL, Fisher DJ. Regional myocardial function is not affected by severe coronary depressurization provided coronary blood flow is maintained. J Am Coll Cardiol. 1985;5(4):948-955.
- Meuwissen M, Siebes M, Chamuleau SA, et al. Hyperemic stenosis resistance index for evaluation of functional coronary lesion severity. Circulation. 2002;106(4):441-446.
- Manders WT, Vatner SF. Effects of sodium pentobarbital anesthesia on left ventricular function and distribution of cardiac output in dogs, with particular reference to the mechanism for tachycardia. Circ Res. 1976;39(4):512-517.
- Merin RG, Verdouw PD, de Jong JW. Myocardial functional and metabolic responses to ischemia in swine during halothane and fentanyl anesthesia. Anesthesiology. 1982;56(2):84-92.
- Triana JF, Li XY, Jamaluddin U, Thornby JI, Bolli R. Postischemic myocardial “stunning.” Identification of major differences between the open-chest and the conscious dog and evaluation of the oxygen radical hypothesis in the conscious dog. Circ Res. 1991;69(3):731-747.
- Vatner SF, Higgins CB, Patrick T, Franklin D, Braunwald E. Effects of cardiac depression and of anesthesia on the myocardial action of a cardiac glycoside. J Clin Invest. 1971;50(12):2585-2595.
- Zimpfer M, Manders WT, Barger AC, Vatner SF. Pentobarbital alters compensatory neural and humoral mechanisms in response to hemorrhage. Am J Physiol. 1982;243(5):H713-H721.
- Hickey RF, Cason BA, Shubayev I. Regional vasodilating properties of isoflurane in normal swine myocardium. Anesthesiology. 1994;80(3):574-581.
- Vatner SF, Braunwald E. Cardiovascular control mechanisms in the conscious state. N Engl J Med. 1975;293(19):970-976.
- Higgins CB, Vatner SF, Franklin D, Braunwald E. Extent of regulation of the heart’s contractile state in the conscious dog by alteration in the frequency of contraction. J Clin Invest. 1973;52(5):1187-1194.
_______________________________________
From the 1School of Dynamic Systems, Mechanical Engineering Program, 2Departments of Internal Medicine and Cardiology, 3Department of Environmental Health, University of Cincinnati, 4Heart Institute, Cincinnati Children’s Hospital Medical Center, 5Deaconess Hospital, Cincinnati, Ohio.
Funding source: This work was supported by Grant-In-Aid of Great Rivers Affiliate and National-Scientific Development Grant of American Heart Association (Grant reference #0755236B and #0335270N).
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted July 6, 2011, provisional acceptance given August 10, 2011, final version accepted October 11, 2011.
Address for correspondence: R.K. Banerjee, PhD, PE, School of Dynamic Systems, Mechanical Engineering Program, 598 Rhodes Hall, PO Box 210072, Cincinnati, OH 45221-0072. Email: Rupak.Banerjee@UC.edu












