ADVERTISEMENT
Early Detection of Chronic Myocardial Ischemia in a Patient Implanted with an ICD Capable of Intracardiac Electrogram Monitoring
Abstract: Although myocardial ischemia monitored by some implantable cardioverter-defibrillators has the potential to improve patient care, no clinical experience with this novel technology has been described yet. We report for the first time ischemic intracardiac ST changes detected in a patient with coronary artery disease.
J INVASIVE CARDIOL 2011;23(12):532-533
Key words: continuous monitoring, myocardial ischemia, ST-segment, implantable cardioverter defibrillator, coronary artery disease
_________________________________________
Intracardiac ST-segment monitoring through implanted devices is an emerging concept to detect early warning signs of myocardial ischemia in order to reduce morbidity and mortality resulting from underlying coronary artery disease and its various complications. Comparative studies have shown that intracardiac electrocardiogram (IEGM) is more sensitive and specific than surface ECG and early experiences have supported the use of intracardiac electrograms to detect acute ischemic events.1-5 Recently, multiprogrammable defibrillators with the ability of continuous IEGM-based ST monitoring through the ventricular lead have been developed. These devices (AnalyST Accel ICD and Fortify ST ICD; St. Jude Medical) continuously measure and store any change in ST segment that might be associated with myocardial ischemia. The unipolar ICD to right ventricular tip is the recording vector used for ST-segment monitoring. If an ST shift is detected, the device will store the episode date/time of onset, the heart rate zone, the duration, and the maximum ST shift recorded. Each stored ST-shift episode has three accompanying electrograms: the baseline IEGM obtained in the previous 72 hours, an IEGM taken at the time of detection of the event (time of onset), and an IEGM that records the largest measured ST-shift during the episode.
To the best of our knowledge, no clinical experience with ICD-based ischemia monitoring has been reported yet. We report for the first time ischemic intracardiac ST changes detected in an ICD-implanted patient with coronary artery disease.
Case Description
 A 75-year-old man with previous coronary artery bypass surgery in 1983 and 1995, previous percutaneous coronary revascularization in 2002 and 2006, and ICD implantation in 2004 for secondary prevention of sudden cardiac death, came to our division in January 2010 for battery depletion of the ICD generator. The ICD was replaced with an AnalyST Accel DR. The procedure was uncomplicated, and he was discharged 2 days later with normal pacing parameters.
A 75-year-old man with previous coronary artery bypass surgery in 1983 and 1995, previous percutaneous coronary revascularization in 2002 and 2006, and ICD implantation in 2004 for secondary prevention of sudden cardiac death, came to our division in January 2010 for battery depletion of the ICD generator. The ICD was replaced with an AnalyST Accel DR. The procedure was uncomplicated, and he was discharged 2 days later with normal pacing parameters.
One month after device implantation, the patient returned to program heart rate ranges and respective ischemia detection thresholds as suggested by the algorithm. At that time, device interrogation did not show any arrhythmic or ischemic events. The patient was provided with a remote monitoring system that had not been used, for technical reasons, during follow-up.
During the last in-clinic visit (September 2010), he reported stress angina; coronary angiography was prescribed, but refused by the patient. On January 2011, he was admitted to the emergency department for unstable angina. The patient reported that episodes of angina had become more frequent and more severe since his last in-clinic visit. At admission, ECG was normal, Troponin T was found increased, and coronary angiography was performed.
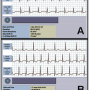
The exam revealed critical stenosis of two saphenous grafts (for obtuse marginal and right coronary artery) that were treated with stent implantation. The two venous grafts were patent in a previous coronary angiography that was performed in 2006. Device interrogation performed after the revascularization procedure showed frequent ST-shift events (n = 141) detected during elevated heart rates. These events had started a few days after the last in-clinic visit, but remained unrecognized until the device interrogation because the patient did not have a remote monitoring system. The analysis of the 5 IEGMs available revealed ST changes suggestive of ischemic events (Figure 1) and the last one had occurred the day before hospital admission.
After revascularization, the patient remained asymptomatic and free of ST events during 8 months of follow-up.
Discussion
Coronary artery disease is a frequent comorbidity as well as a potential cause of unfavorable clinical events in ICD recipients. Early detection of myocardial ischemia and prompt intervention may substantially improve clinical outcomes. However, currently available non-invasive assessment methods of myocardial ischemia do not allow for continuous assessment or monitoring.
Recently, multiprogrammable defibrillators with the ability of continuous IEGM-based ST monitoring through the ventricular lead have been developed. However, no clinical experience with ICD-based ischemia monitoring has been reported yet. To the best of our knowledge, this is the first reported case of myocardial ischemia detected with an ICD capable of intracardiac ST continuous monitoring.
Fishell et al5 recently reported a clinical experience with an intracardiac ischemia monitoring system in 37 high-risk patients. The implanted monitor proved to be useful to detect and alert patients in case of acute ischemic events, thereby reducing alert-to-door time for patients at high risk of recurrent coronary syndromes. Since ICD patients with known coronary artery disease are at a high risk of adverse events, it was reasonable to postulate that ICD-based ischemia monitoring would improve survival among these patients. However, currently available ICDs do not have any patient alert system; therefore, it remains questionable whether a system without a patient alert might be useful in this subset of patients.
In the present report, demand-related ischemia was detected in a patient but remained unrecognized until the next device interrogation. The patient’s inability to use a remote monitoring system definitely influenced clinical outcome. With devices that offer the potential of an internal alert mechanism and the possibility of sending automatic alert messages, both the physician and the patient might be notified at the onset of myocardial ischemia so that appropriate treatment could be promptly instituted. The next generation of system would address the aforementioned limitations; in this way, critical events may be reported earlier, allowing prompt and effective treatment.
Interestingly, the patient reported a reduction of angina threshold during time. Accordingly, the algorithm initially reported ischemic ST events during high heart rates and subsequently ST events were also detected during lower heart rates. However, in this patient, the clinical manifestation of myocardial ischemia preceded ST-segment modification detected by the algorithm. This last point suggests that even though myocardial ischemia has been properly detected, the sensitivity and specificity of the algorithm still require validation.
This report demonstrates that ICD-based ischemia monitoring is a promising technique; nevertheless, potential improvement of the algorithm might promote an early and preclinical detection of myocardial ischemia so that appropriate treatment can be promptly instituted.
References
- Theres H, Stadler RW, Stylos L, et al. Comparison of electrocardiogram and intrathoracic electrogram signals for detection of ischemic ST-segment changes during normal sinus and ventricular paced rhythms. J Cardiovasc Electrophysiol. 2002;13(10):990-995.
- Fischell TA, Fischell DR, Fischell RE, et al. Potential of an intracardiac electrogram for the rapid detection of coronary artery occlusion. Cardiovasc Revasc Med. 2005;6(1):14-20.
- Asbach S, Weiss I, Wenzel B, Bode C, Zehender M. Intrathoracic far-field electrocardiogram allows continuous monitoring of ischemia after total coronary occlusion. Pacing Clin Electrophysiol. 2006;29(12):1334-1340.
- Fischell TA, Fischell DR, Fischell RE, Virmani R, DeVries JJ, Krucoff MW. Real-time detection and alerting for acute ST-segment elevation myocardial ischemia using an implantable, high-fidelity, intracardiac electrogram monitoring system with long-range telemetry in an ambulatory porcine model. J Am Coll Cardiol. 2006;48(11):2306-2314.
- Fischell TA, Fischell DR, Avezum A, et al. Initial clinical results using intracardiac electrogram monitoring to detect and alert patients during coronary plaque rupture and ischemia. J Am Coll Cardiol. 2010;56(14):1089–1098.
_________________________________________
From the University of Rome “Tor Vergata,” Department of Internal Medicine, Division of Cardiology, Rome, Italy.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Giovanni B. Forleo received lecture fees from St. Jude Medical. No other authors report any conflicts of interest regarding the content herein.
Manuscript submitted August 23, 2011, provisional acceptance given September 7, 2011, final version accepted September 19, 2011.
Address for correspondence. Lida P. Papavasileiou, MD. Division of Cardiology, University Hospital of Tor Vergata, Viale Oxford, 81. 00133, Roma, Italy. Email: lidapieretta@hotmail.com












