Drug-Eluting vs Bare-Metal Stents in Patients With Chronic Kidney Disease and Coronary Artery Disease: Insights From a Systematic Review and Meta-Analysis
Abstract: Background. Most drug-eluting stent (DES) trials have excluded patients with chronic kidney disease (CKD). The efficacy of DES implantation in patients with CKD is therefore not known. Objectives. To evaluate the outcomes with DES vs bare-metal stent (BMS) implantation in patients with CKD. Methods and Results. MEDLINE, EMBASE, and CENTRAL were searched for studies including at least 100 patients with CKD (estimated glomerular filtration rate ≤60 mL/min/1.73 m2 or on dialysis) treated with DES or BMS and followed for at least 1 month and reporting outcomes of all-cause mortality, cardiovascular (CV) mortality, myocardial infarction (MI), target-vessel revascularization (TVR), and stent thrombosis (ST). Thirty-one studies (5 randomized) with 91,817 participants (49,081 DES and 42,736 BMS) fulfilled the inclusion criteria. DES was associated with lower all-cause mortality (relative risk [RR], 0.77; 95% confidence interval [CI], 0.71-0.84), CV mortality (RR, 0.51; 95% CI, 0.38-0.70), MI (RR, 0.90; 95% CI, 0.86-0.95), TVR (RR, 0.61; 95% CI, 0.47-0.80), and numerically lower ST (RR,0.75; 95% CI, 0.55-1.01) when compared with BMS. Analysis by study type (RCTs vs non-RCTs) showed similar results for most outcomes (Pinteraction>.05) except all-cause mortality, where there was no difference between DES vs BMS in RCTs (Pinteraction=.04). The effects were greater with 2nd-generation DES vs BMS (for example, ST: RR, 0.38; 95% CI, 0.20-0.72). Conclusions. In patients with CKD, the available evidence, largely from observational studies, suggests significantly fewer events with DES vs BMS with even a lower ST rate with 2nd-generation DES. These findings should be tested in large, randomized trials.
J INVASIVE CARDIOL 2018;30(1):10-17. Epub 2017 September 15.
Key words: coronary artery disease, chronic kidney disease, percutaneous coronary intervention, drug-eluting stent, bare-metal stent
The development of bare-metal stent (BMS) implantation was a major advance over balloon angioplasty in the treatment of coronary artery disease (CAD), preventing early arterial recoil and providing a non-surgical method for the treatment of dissections. However, 10%-20% patients who receive BMS develop restenosis.1-3 Restenosis is higher in patients with chronic kidney disease (CKD) due to increased inflammation, higher prevalence of diabetes, and presence of calcification.4 Drug-eluting stent (DES) implantation was developed as a means to decrease neointimal proliferation, which contributes to the development of restenosis. However, DES implantation necessitates prolonged antiplatelet therapy when compared with BMS implantation. This can be problematic, as patients with CKD are also at a significantly increased risk of bleeding.5,6 Furthermore, the recently published NORSTENT trial failed to show a benefit of newer-generation DES over BMS with regard to myocardial infarction (MI) and all-cause mortality among a general population.7 It is unclear how many CKD patients were included in NORSTENT given that patients with serious medical conditions other than CAD and with a life expectancy of <5 years were excluded from the trial. In fact, patients with CKD are routinely excluded from randomized control trials (RCTs).8,9 As such, it is not clear whether patients with CKD and CAD derive benefit from DES over BMS and whether the benefit is merely restricted to reducing restenosis or a broader reduction in mortality and MI. Therefore, the objective of this study was to perform a meta-analysis of published studies to compare the outcomes of revascularization with DES and BMS in patients with CKD.
Methods
Eligibility criteria. We conducted MEDLINE, EMBASE, and CENTRAL searches (until October 2016) using the MeSH terms “kidney failure, chronic” or “renal insufficiency, chronic” and “coronary artery disease.” No language restrictions were applied. Relevant published reviews, meta-analyses, and original articles were reviewed to find other eligible studies. Eligible studies had to fulfill the following criteria: (1) include at least 100 patients who have estimated glomerular filtration rate (eGFR) ≤60 mL/min/1.73 m2 or are on dialysis and have concomitant CAD; (2) compare two stent groups – DES and BMS; 3) have a follow-up duration of at least 1 month; and (4) include any of the outcomes of interest described below.
Selection and quality assessment. The results of the searches were compiled using EndNote X7.7 software. After removal of duplicates, two reviewers (AV and SK) independently screened each study by title and abstract for inclusion. Those studies that qualified for full text review were again reviewed independently by two reviewers for inclusion into the analysis. The reviewers performed data abstraction and independently assessed the included studies for sources of systematic bias, as per the guidelines recommended by the Cochrane Handbook for Systematic Reviews of Interventions. Any disagreements between reviewers were resolved by consensus and, if necessary, adjudicated by a third reviewer (SB).
Data extraction and outcome. Data abstracted included study characteristics, patient characteristics, and details regarding the two treatment groups. The primary outcome of the study was all-cause mortality. Secondary outcome measures were cardiovascular (CV) mortality, recurrent MI, target-lesion revascularization (TLR), and target-vessel revascularization (TVR). Trial quality was assessed via the Newcastle-Ottawa scale (NOS) for assessing the quality of non-RCT studies in meta-analyses and as per the Cochrane Handbook for Systematic Reviews of Interventions for RCTs.10,11
Statistical analysis. Meta-analysis was performed using RevMan v. 5.3.5 (Cochrane Collaboration). The pooled effect estimate was calculated for all included studies using the Mantel-Haenszel method with random-effects model. Heterogeneity was assessed by estimating the I2 statistic to determine the proportion of variation attributable to heterogeneity among studies (I2 >50% suggesting significant heterogeneity). The small-studies effect was estimated visually by funnel plots. Analyses were performed comparing all-cause mortality, CV mortality, MI, TVR, and TLR in DES vs BMS. Subgroup analyses were conducted after stratifying the studies by the following: (1) cohort enrolled: mixed CKD cohort vs end-stage renal disease (ESRD)-only cohort; (2) type of DES compared: 1st-generation DES vs 2nd-generation DES; and (3) study type: RCT vs non-RCT. The differences between subgroups were tested by a P-value for interaction, with Pinteraction<.05 used to denote statistical significance either qualitatively or quantitatively. Further subgroup analyses were performed comparing outcomes with 1st-generation DES vs 2nd-generation DES.
Results
Study selection. We identified 3774 unique records that were reviewed based on title and/or abstract for relevance, from which 175 studies were reviewed in depth; 31 studies were included in the final analysis after meeting the aforementioned inclusion criteria. The included studies were a mix of RCTs and non-RCTs. Data obtained from RCTs were subgroup analyses of the CKD cohorts enrolled in these trials, as no available RCTs were specifically designed to study CKD patients.
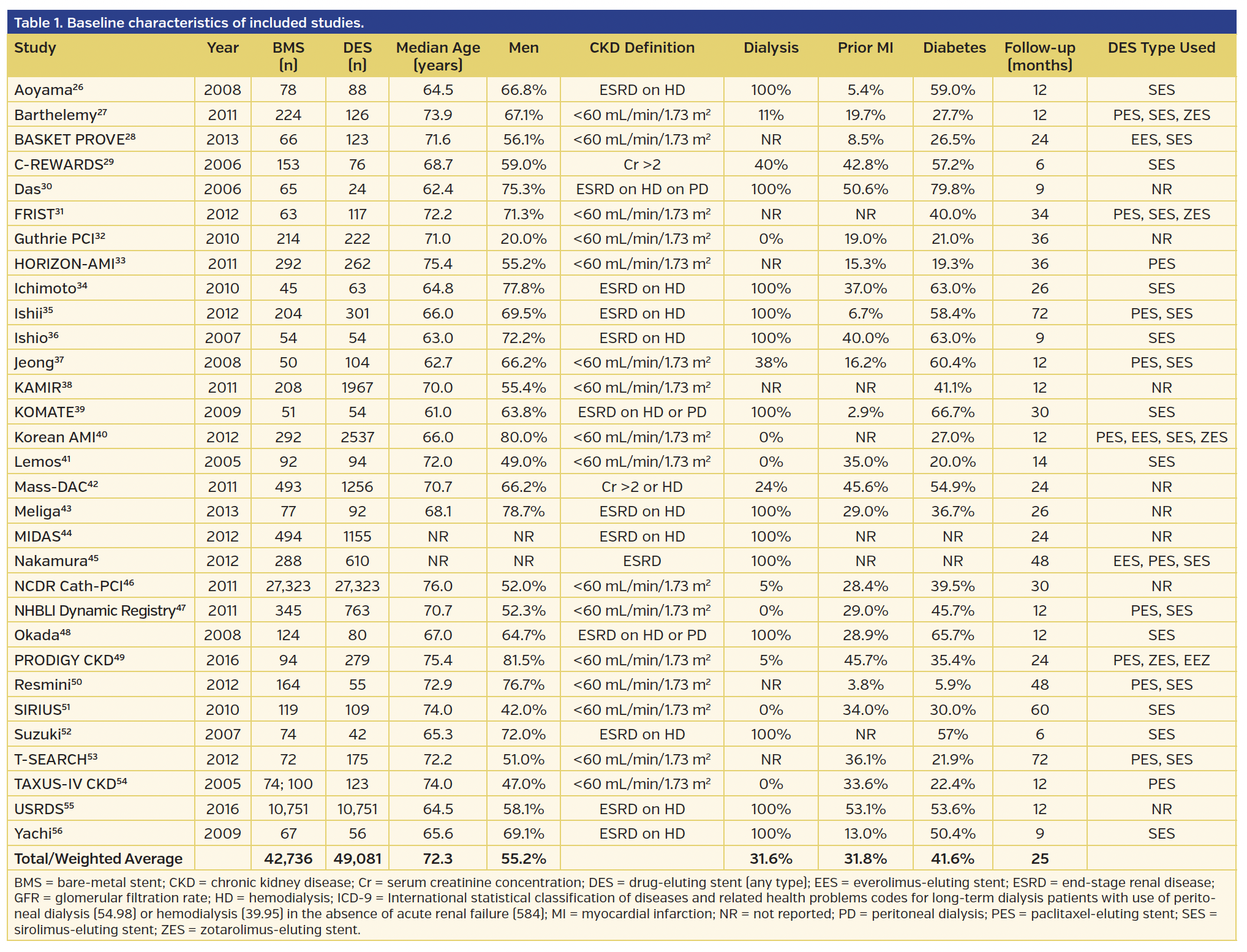
Baseline characteristics. The baseline characteristics of the included studies are summarized in Table 1. The 31 studies included 91,817 patients, of which 49,081 received DES (1905 2nd-generation DES) and 42,736 received BMS. The mean follow-up was 2.1 years. Of the 31 studies, 13 studies, all non-RCTs (21,095 patients), exclusively included ESRD-only patients undergoing dialysis with the remainder of the studies with heterogeneous cohort of patients with CKD (5% of which were ESRD) as outlined in Table 1. Overall, there were only 5 RCTs with 1567 patients, which constituted 1.7% of the cohort studied. No RCT-reported data in patients with ESRD only.
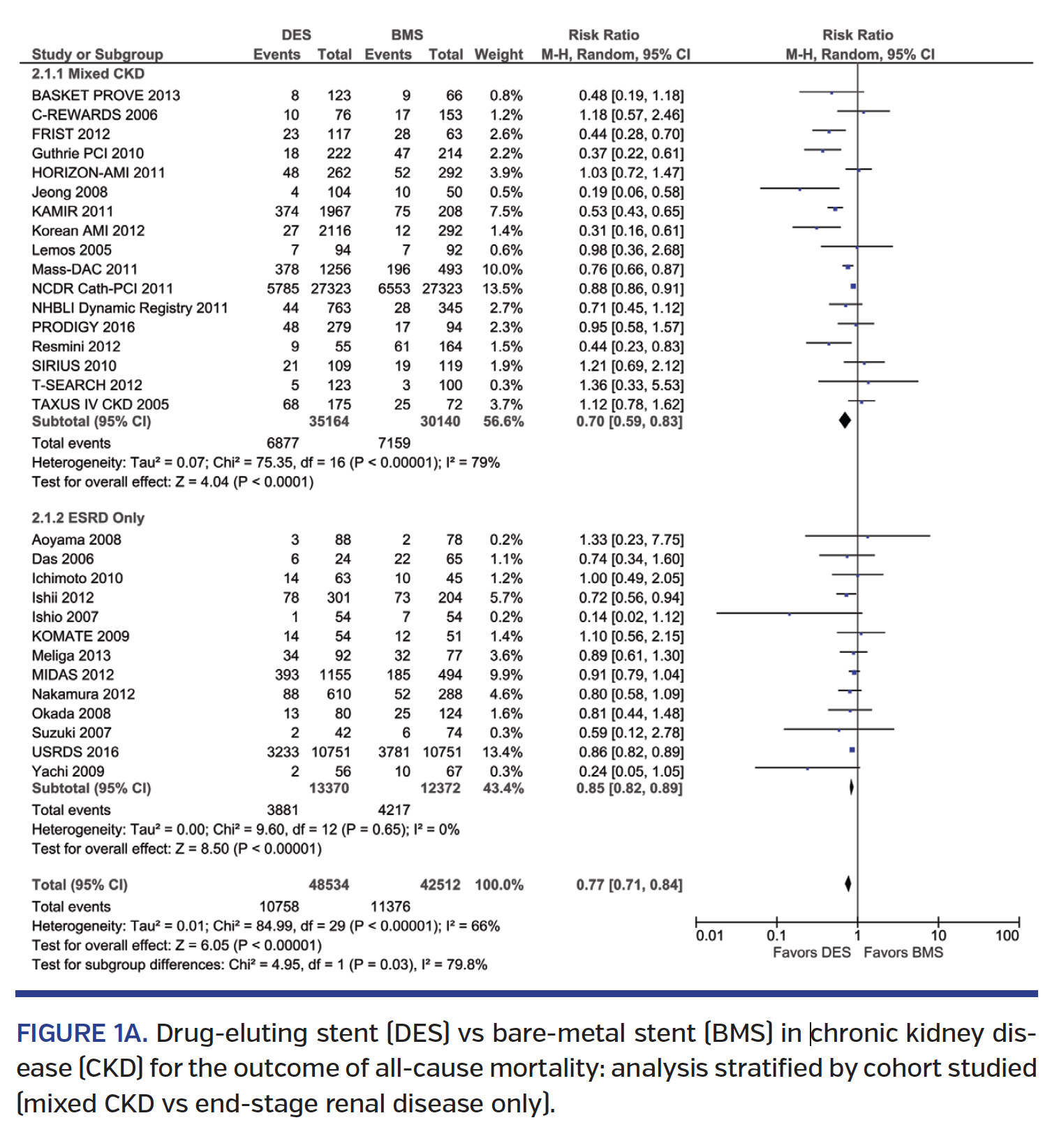
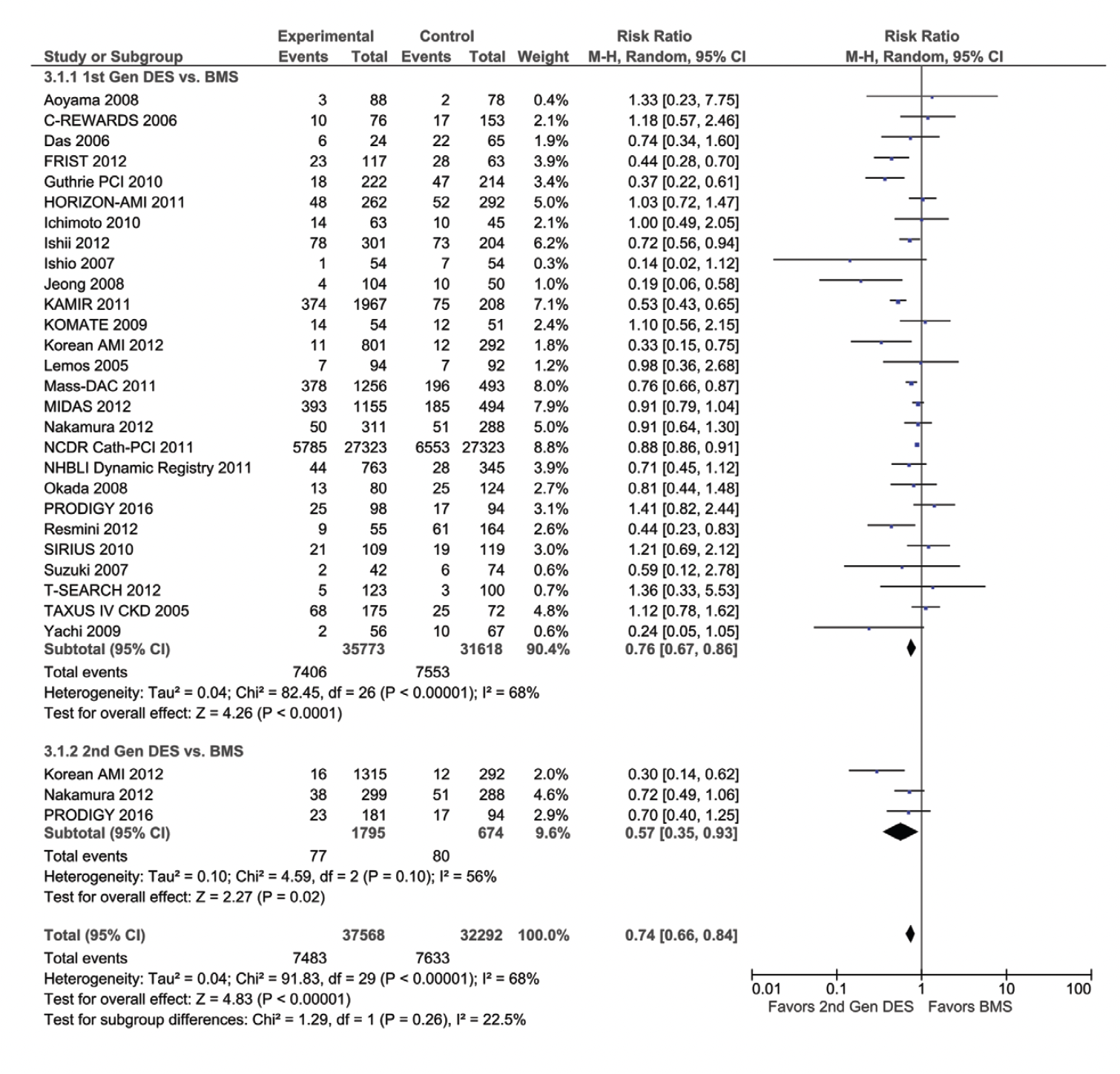
Mortality. Revascularization with DES was associated with a 23% lower mortality (risk ratio [RR], 0.77; 95% confidence interval [CI], 0.71-0.84) when compared with BMS (Figure 1A), driven by a 30% lower mortality among the mixed CKD cohort (RR, 0.70; 95% CI, 0.59-0.83), but only a 15% lower mortality (RR, 0.85; 95% CI, 0.82-0.89) in the ESRD-only cohort (Pinteraction=.03) (Figure 1A). The lower mortality was seen with both 1st-generation and 2nd-generation DES vs BMS, with similar results in the mixed CKD subgroup and in the ESRD-only cohort (Pinteraction=.26) (Figure 1B). In the studies comparing 1st-generation vs 2nd-generation DES, 2nd-generation DES was associated with a 31% lower mortality (RR, 0.69; 95% CI, 0.50-0.96) (Supplemental Figure S1A; all supplemental figures available at end of Results Section). Subgroup analysis performed by study type showed lower mortality in non-RCT studies but not in RCT trials, although the RCT trial subgroup was small (Pinteraction=.04) (Supplemental Figure S1B). Significant heterogeneity was observed in the mortality analyses.
DES implantation was associated with a 49% lower CV mortality (RR, 0.51; 95% CI, 0.38-0.70), with similar results in the mixed CKD subgroup and in the ESRD-only cohort (Pinteraction=.14) (Supplemental Figure S2A). Results were consistent with both 1st-generation and 2nd-generation DES vs BMS, although the magnitude was larger with 2nd-generation DES (Pinteraction=.04) (Supplemental Figure S2B). In the studies comparing 1st-generation vs 2nd-generation DES, there was no difference in CV mortality (RR, 0.61; 95% CI, 0.21-1.73), albeit the sample size was small (Supplemental Figure S2C). Subgroup analysis performed by study type showed similar results in non-RCTs and RCTs (Pinteraction=.42) (Supplemental Figure S2D). Significant heterogeneity was observed in the CV mortality analyses.
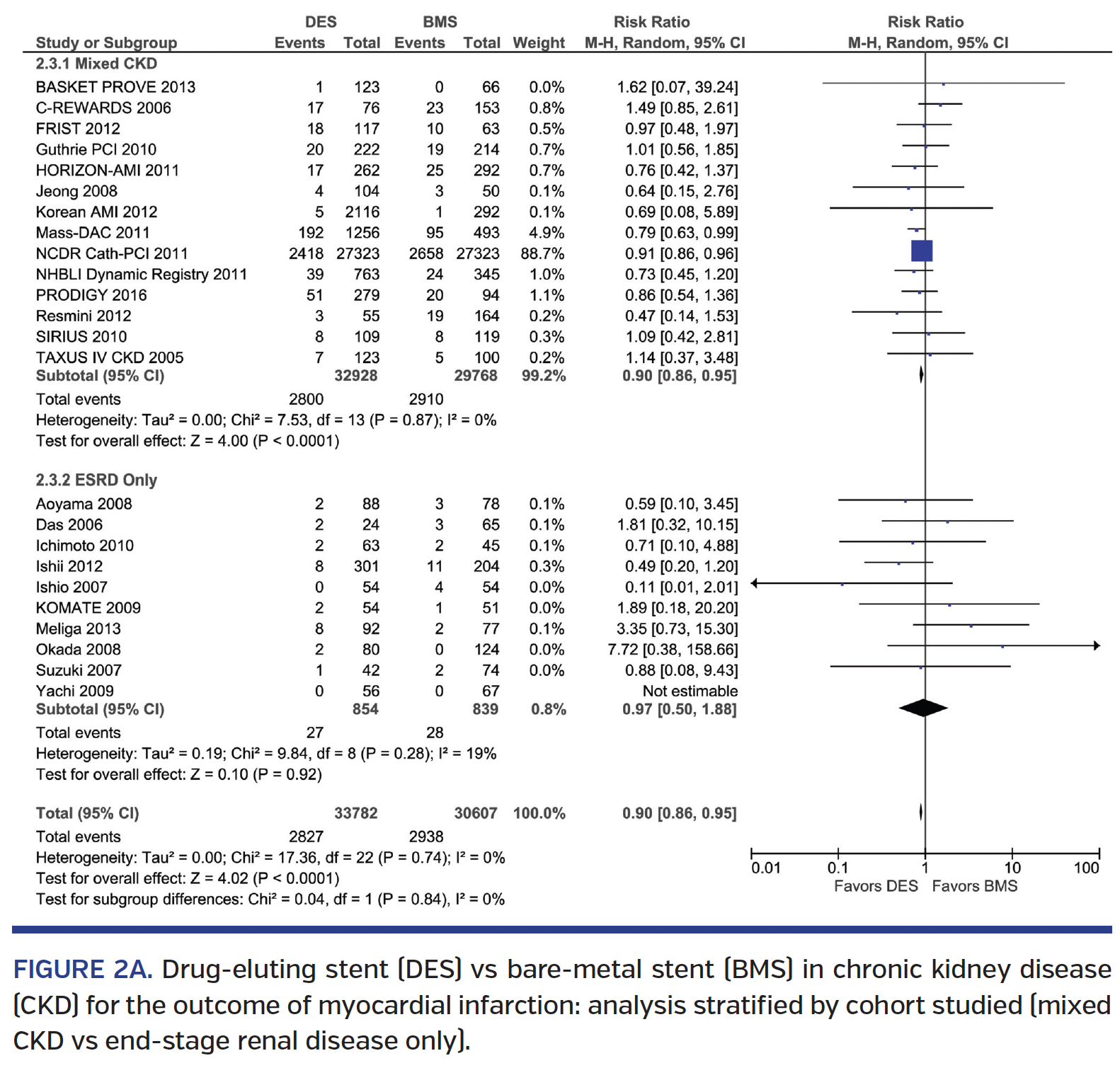
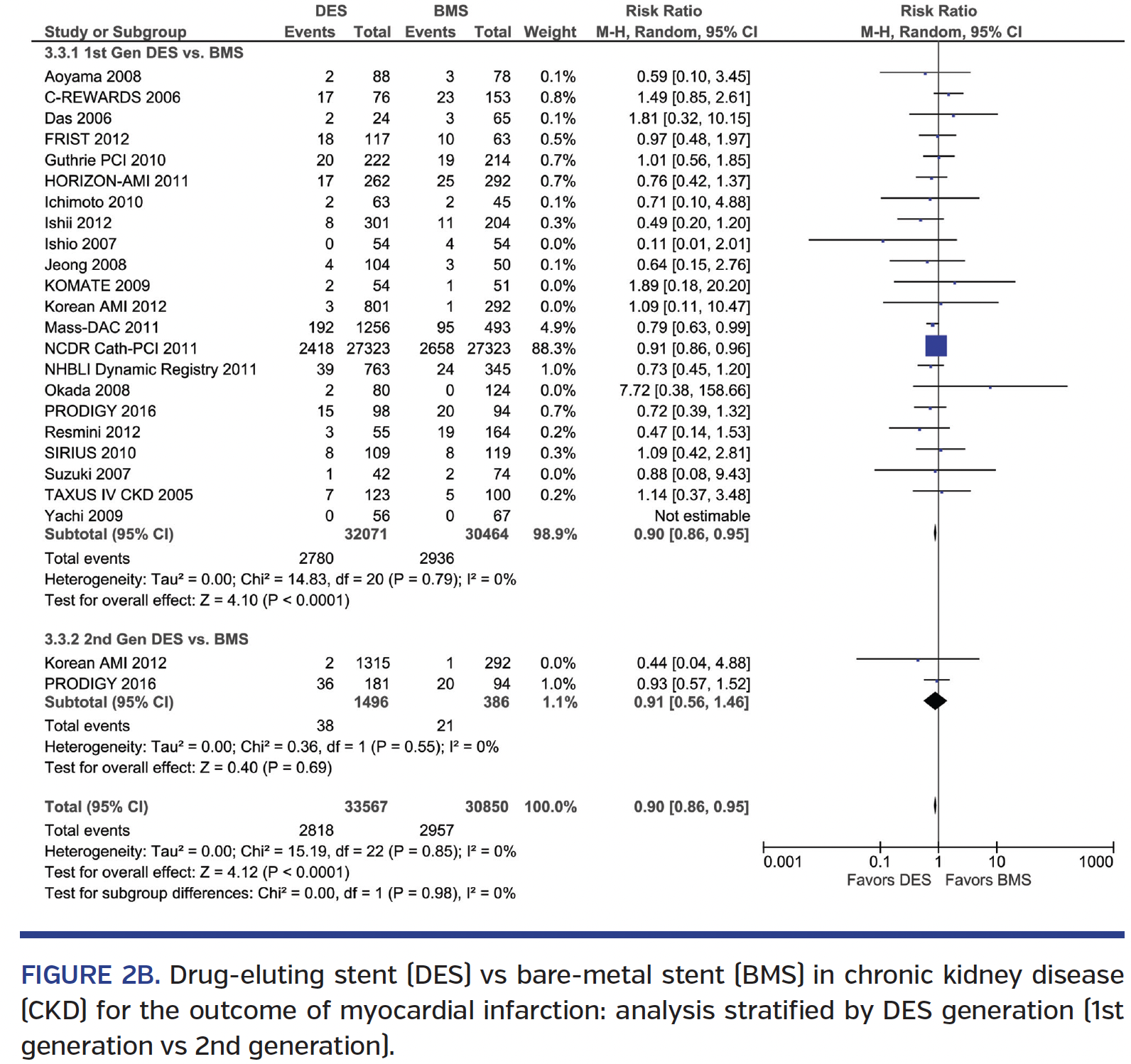
Myocardial infarction. DES implantation was associated with a 10% lower MI when compared to BMS (RR, 0.90; 95% CI, 0.86-0.95), with similar results in the mixed CKD subgroup and in the ESRD-only cohort (Pinteraction=.84) (Figure 2A). Results were consistent with both 1st-generation and 2nd-generation DES vs BMS (Pinteraction=.98) (Figure 2B). Comparison of 1st-generation vs 2nd-generation DES showed similar MI rates (RR, 1.00; 95% CI, 0.39-2.59) (Supplemental Figure S3A). Analysis by study type showed similar results in both non-RCTs and RCTs (Pinteraction=.86) (Supplemental Figure S3B).
Target-vessel/target-lesion revascularization. DES was associated with a 39% lower TVR rate (RR, 0.61; 95% CI, 0.47-0.80) with similar results in the mixed CKD subgroup and in the ESRD-only cohort (Pinteraction=.90) (Supplemental Figure S4A). Results were consistent with both 1st-generation and 2nd-generation DES vs BMS (Pinteraction=.21) (Supplemental Figure S4B). There was no difference seen in a direct comparison of a small sample size of 1st-generation vs 2nd-generation DES patients with regard to TVR (RR, 0.84; 95% CI, 0.28-2.53) (Supplemental Figure S4C). Analysis by study type showed similar results in both non-RCTs and RCTs (Pinteraction=.68) (Supplemental Figure S4D). Some TVR analyses exhibited significant heterogeneity.
Similarly, DES had a 28% lower rate of TLR (RR, 0.72; 95% CI, 0.58-0.90), with similar results in the mixed CKD subgroup and in the ESRD-only cohort (Pinteraction=.17) (Supplemental Figure S5A). Results were consistent with both 1st-generation and 2nd-generation DES vs BMS (Pinteraction=.86) (Supplemental Figure S5B). There was no difference when comparing a small sample size of 1st-generation vs 2nd-generation DES with regard to TLR (RR, 0.59; 95% CI, 0.11-3.19) (Supplemental Figure S5C). Analysis by study type showed similar results in both non-RCTs and RCTs (Pinteraction=.69) (Supplemental Figure S5D).
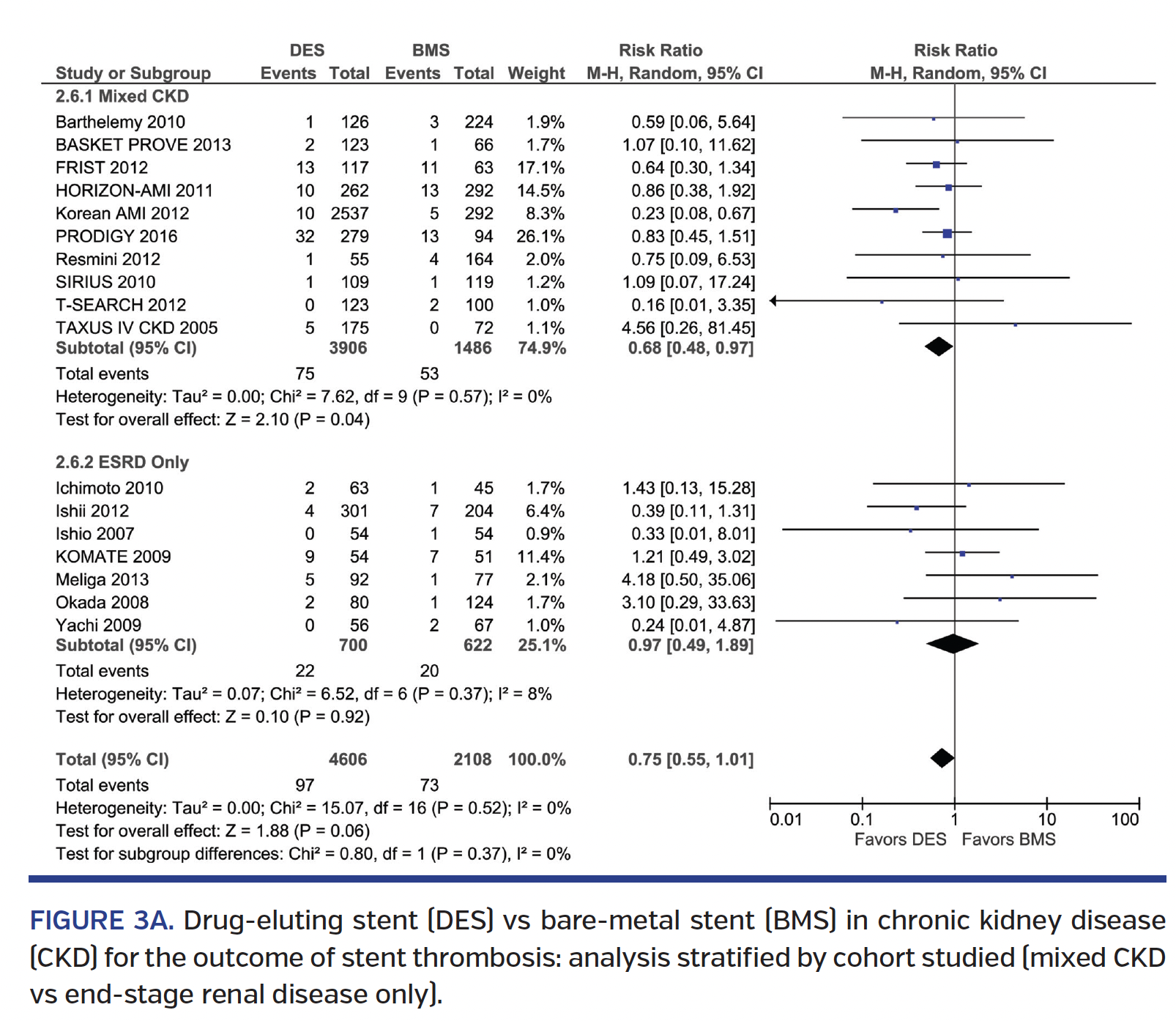
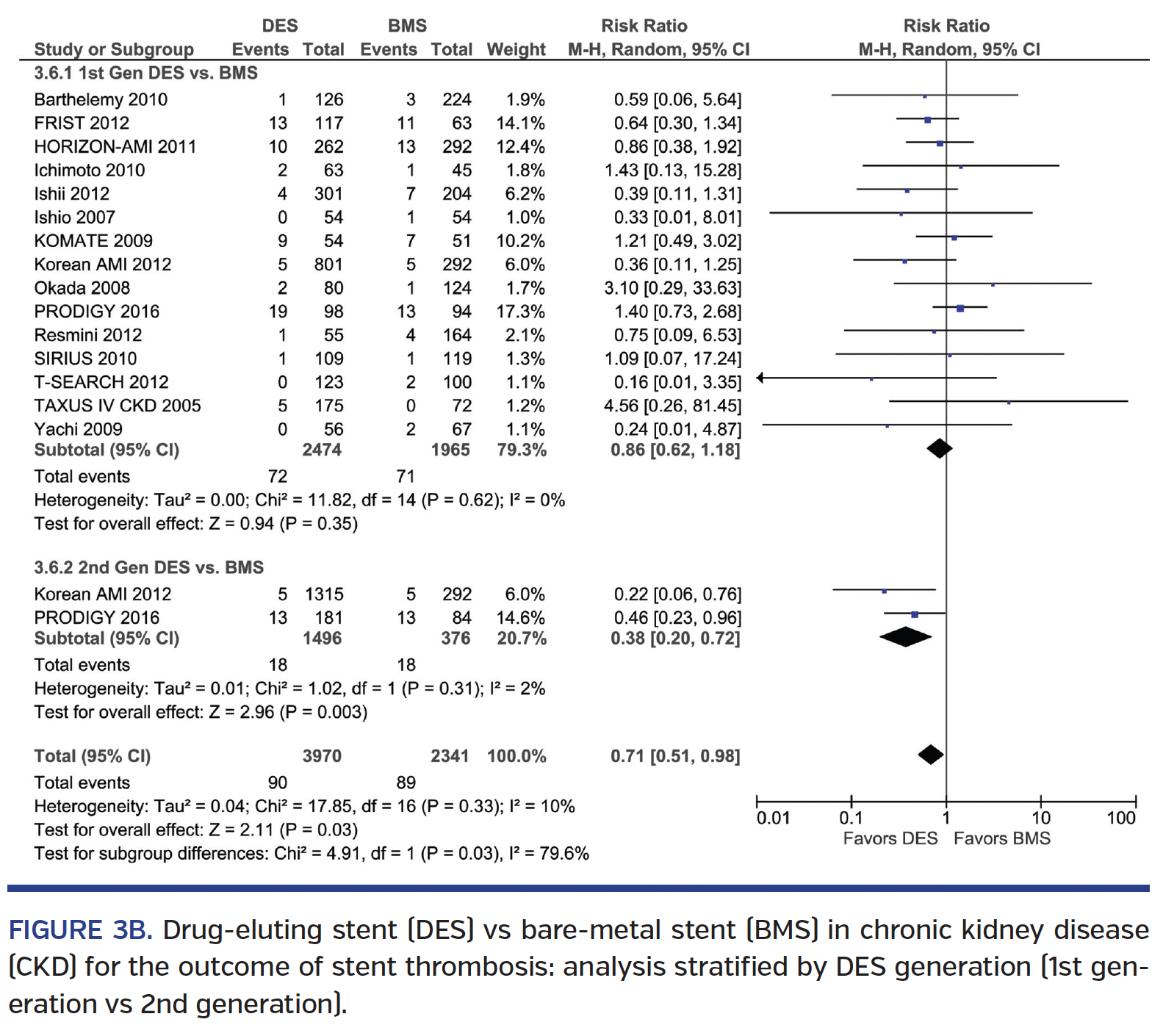
 Stent thrombosis. DES was associated with a trend toward lower ST rate over BMS (RR, 0.75; 95% CI, 0.55-1.01), with no interaction based on ESRD status (Pinteraction=.37) (Figure 3A). Subgroup analysis revealed no difference in ST seen among 1st-generation DES vs BMS (RR, 0.86; 95% CI, 0.62-1.18), but a 62% lower ST rate with 2nd-generation DES vs BMS (RR, 0.38; 95% CI, 0.20-0.72) (Pinteraction=.03) (Figure 3B). Direct comparison of 1st-generation vs 2nd-generation DES studies showed a trend toward a 64% lower ST rate with 2nd-generation DES (RR, 0.34; 95% CI, 0.10-1.11) (Supplemental Figure S6A). Subgroup analysis of RCT and non-RCTs revealed no significant interaction (Pinteraction=.70) (Supplemental Figure S6B). There was significant heterogeneity seen in some ST analyses.
Stent thrombosis. DES was associated with a trend toward lower ST rate over BMS (RR, 0.75; 95% CI, 0.55-1.01), with no interaction based on ESRD status (Pinteraction=.37) (Figure 3A). Subgroup analysis revealed no difference in ST seen among 1st-generation DES vs BMS (RR, 0.86; 95% CI, 0.62-1.18), but a 62% lower ST rate with 2nd-generation DES vs BMS (RR, 0.38; 95% CI, 0.20-0.72) (Pinteraction=.03) (Figure 3B). Direct comparison of 1st-generation vs 2nd-generation DES studies showed a trend toward a 64% lower ST rate with 2nd-generation DES (RR, 0.34; 95% CI, 0.10-1.11) (Supplemental Figure S6A). Subgroup analysis of RCT and non-RCTs revealed no significant interaction (Pinteraction=.70) (Supplemental Figure S6B). There was significant heterogeneity seen in some ST analyses.
Discussion
Our results, based on data from RCTs and non-RCTs, indicate lower mortality, MI, TLR, and TVR rates, as well as borderline lower ST rates, with DES vs BMS in patients with CKD. The effect was greater with 2nd-generation DES for mortality and ST.
According to the Centers for Disease Control’s National Health and Nutrition Examination Survey (NHANES), there are over 20 million adults with CKD in the United States.12 Patients with CKD have an increased incidence of CAD, with some estimating that the majority of ESRD population have CAD.13,14 The reason for these findings, albeit incompletely understood, is thought to be due to increased prevalence of traditional CV risk factors and also the presence of inflammation and oxidative stress leading to endothelial dysfunction.15,16
Management of patients with CAD and CKD continues to be challenging. Many trials have systematically excluded patients with CKD.8,9 Furthermore, revascularization of patients with CKD presents its own set of challenges due to complex lesions, diffuse disease, extensive calcification, small vessel diameters, high prevalence of diabetes, and multivessel disease.17 Patients with CKD presenting with an acute MI are much less likely to undergo PCI.18 The patients who do undergo PCI experience more bleeding complications, compelling some operators to choose BMS implantation. On the other hand, patients with CKD experience higher restenosis rates, with as many as 19% of ESRD patients developing restenosis at 6 months.19 This is thought to be due to chronic inflammation and oxidative stress, which may contribute to accelerated neointimal hyperplasia and smooth muscle cell proliferation, which in turn may contribute to increased rates of in-stent restenosis.20,21 The antiproliferative properties of DESs attempt to mitigate these effects at a cost of need for prolonged antiplatelet therapy due to concerns for ST.22 Patients with CKD are also at a three-fold higher risk of ST compared to non-CKD controls, possibly due in part to the same underlying proinflammatory state.23 As a result, the risk calculators such as DAPT and PRECISE-DAPT include renal insufficiency as a risk factor for both bleeding and ischemic events.24,25
Our study, with data from 91,817 patients with CKD, showed several interesting findings. First, revascularization with DES was associated with lower mortality, MI, and need for revascularization when compared with BMS. Second, subgroup analyses by stent type (1st-generation and 2nd-generation DES) showed that the benefit was greater with 2nd-generation DES for a few of the outcomes (such as mortality and ST). Third, subgroup analyses by study type (RCTs vs non-RCTs) showed similar results for most outcomes (Pinteraction>.05) except for all-cause mortality, where there was no difference between DES vs BMS in RCTs (Pinteraction=.04). Of note, there were only 5 RCTs included and the number of patients comprised <2% of the population studied. As such, even though the test for interaction (RCTs vs non-RCTs) was not significant, analysis of RCTs only showed a statistically significant difference for TVR and no other outcomes. Finally, subgroup analyses based on study cohort (mixed CKD vs ESRD only) showed similar results for most outcomes (Pinteraction>.05) except for all-cause mortality, where there was only a quantitative difference, with 15% lower mortality with DES vs BMS in the ESRD-only cohort (Pinteraction=.04).Of note, in the subgroup of studies in the ESRD-only patients, DES had no statistically significant lower MI or repeat revascularization rates, although the test for interaction was negative.
There could be a number of reasons for the attenuated benefit of DES among the ESRD cohort. Patients with ESRD are prone to excessive calcification, which may result in under-deployment of stents. This, in turn, may increase the risk of restenosis and ST in both DES and BMS groups. Alternatively, any of the benefits of DES implantation may be offset by the risk of increased bleeding in ESRD. Moreover, patients with ESRD also die of non-CV causes, leading to an attenuation of the effect of stents on all-cause mortality. Finally, the subgroup on ESRD-only patients was an analysis of a smaller patient subgroup that may be potentially under-powered to detect differences between DES and BMS.
Study limitations. There are a number of limitations in the existing body of evidence to guide stent choice in patients with CKD and CAD. Our findings are based on the meta-analysis of a mix of RCT and non-RCT studies and likely suffers from selection and ascertainment bias. As in other meta-analyses, given the paucity of data, we did not adjust for compliance to assigned treatment, or the medications used. It is therefore unknown how many patients received dual-antiplatelet therapy, the duration of dual-antiplatelet therapy, or the type of P2Y12 inhibitor used in these patients. Additionally, there was significant heterogeneity in many of the analyses, with I2 >50% due to the differences in the included study design, patient population, aggressiveness of medical therapy, PCI techniques, and follow-up. These variables could not be accounted for without individual patient-level data.
Conclusion
In patients with CKD and CAD, data from largely non-RCTs suggest lower mortality, MI, and revascularization rates with DES when compared with BMS, with greater benefit with 2nd-generation DES compared to BMS for certain outcomes (such as ST). The results were consistently seen in subgroup analyses based on study type (RCTs vs non-RCTs) and based on cohort studied (mixed CKD vs ESRD only). Larger RCTs are needed to test these findings.
See .pdf for supplemental figures.
References
1. Cutlip DE, Chauhan MS, Baim DS, et al. Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J Am Coll Cardiol. 2002;40:2082-2089.
2. Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331:496-501.
3. Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994;331:489-495.
4. Best PJM, Lennon R, Ting HH, et al. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2002;39:1113-1119.
5. Kaufman JS, O’Connor TZ, Zhang JH, et al. Randomized controlled trial of clopidogrel plus aspirin to prevent hemodialysis access graft thrombosis. J Am Soc Nephrol. 2003;14:2313-2321.
6. Pavord S, Myers B. Bleeding and thrombotic complications of kidney disease. Blood Reviews. 2011;25:271-278.
7. Bonaa KH, Mannsverk J, Wiseth R, et al. Drug-eluting or bare-metal stents for coronary artery disease. N Engl J Med. 2016;375:1242-1252.
8. Charytan D, Kuntz RE. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Internat. 2006;70:2021-2030.
9. Ishida JH, Johansen KL. Exclusion of patients with kidney disease from cardiovascular trials. JAMA Intern Med. 2016;176:124-125.
10. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
11. Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. 2011.
12. Centers for Disease Control and Prevention. Chronic Kidney Disease Surveillance System—United States. Available at https://www.cdc.gov/ckd. Accessed 2015.
13. Daly C. Is early chronic kidney disease an important risk factor for cardiovascular disease?: A background paper prepared for the UK consensus conference on early chronic kidney disease. Nephrol Dial Transplant. 2007;22:ix19-ix25.
14. Ohtake T, Kobayashi S, Moriya H, et al. High prevalence of occult coronary artery stenosis in patients with chronic kidney disease at the initiation of renal replacement therapy: an angiographic examination. J Am Soc Nephrol. 2005;16:1141-1148.
15. van Guldener C, Janssen MJ, Lambert J, Steyn M, Donker AJ, Stehouwer CD. Endothelium-dependent vasodilatation is impaired in peritoneal dialysis patients. Nephrol Dial Transplant. 1998;13:1782-1786.
16. van Guldener C, Lambert J, Janssen MJ, Donker AJ, Stehouwer CD. Endothelium-dependent vasodilatation and distensibility of large arteries in chronic haemodialysis patients. Nephrol Dial Transplant. 1997;12(Suppl 2):14-18.
17. Cai Q, Mukku VK, Ahmad M. Coronary artery disease in patients with chronic kidney disease: a clinical update. Curr Cardiol Rev. 2013;9:331-339.
18. Coats WC, Baig SZ, Alpert MA, Aggarwal K. Management of coronary artery disease in patients with chronic kidney disease. Adv Perit Dial. 2009;25:125-128.
19. Attallah N, Yassine L, Fisher K, Yee J. Risk of bleeding and restenosis among chronic kidney disease patients undergoing percutaneous coronary intervention. Clin Nephrol. 2005;64:412-418.
20. Farb A, Weber DK, Kolodgie FD, Burke AP, Virmani R. Morphological predictors of restenosis after coronary stenting in humans. Circulation. 2002;105:2974-2980.
21. Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648-658.
22. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082-1115.
23. Miao Y, Yu-Jie Z, Zhi-Jian W, et al. Chronic kidney disease and the risk of stent thrombosis after percutaneous coronary intervention with drug-eluting stents. Catheter Cardiovasc Interv. 2012;80:361-367.
24. Yeh RW, Secemsky EA, Kereiakes DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735-1749.
25. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025-1034.
26. Aoyama T, Ishii H, Toriyama T, et al. Sirolimus-eluting stents vs bare metal stents for coronary intervention in Japanese patients with renal failure on hemodialysis. Circ J. 2008;72:56-60.
27. Barthelemy O, Helft G, Silvain J, et al. One-year clinical outcomes in patients with chronic renal failure treated by percutaneous coronary intervention with drug-eluting stent. Arch Cardiovasc Dis. 2011;104:604-610.
28. Wanitschek M, Pfisterer M, Hvelplund A, et al. Long-term benefits and risks of drug-eluting compared to bare-metal stents in patients with versus without chronic kidney disease. Int J Cardiol. 2013;168:2381-2388. Epub 2013 Feb 27.
29. Kuchulakanti PK, Torguson R, Chu WW, et al. Impact of chronic renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary intervention with sirolimus-eluting stents versus bare metal stents. Am J Cardiol. 2006;97:792-797.
30. Das P, Moliterno DJ, Charnigo R, et al. Impact of drug-eluting stents on outcomes of patients with end-stage renal disease undergoing percutaneous coronary revascularization. J Invasive Cardiol. 2006;18:405-408.
31. Kersting S, Grumann T, Hummel J, Hauschke D, Bode C, Hehrlein C. Impact of chronic kidney disease on long-term clinical outcomes after percutaneous coronary intervention with drug-eluting or bare-metal stents. Critical Pathways Cardiol. 2012;11:152-159.
32. Shenoy C, Boura J, Orshaw P, Harjai KJ. Drug-eluting stents in patients with chronic kidney disease: a prospective registry study. PloS One. 2010;5:e15070.
33. Saltzman AJ, Stone GW, Claessen BE, et al. Long-term impact of chronic kidney disease in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 2011;4:1011-1019.
34. Ichimoto E, Kobayashi Y, Iijima Y, Kuroda N, Kohno Y, Komuro I. Long-term clinical outcomes after sirolimus-eluting stent implantation in dialysis patients. Int Heart J. 2010;51:92-97.
35. Ishii H, Toriyama T, Aoyama T, et al. Percutaneous coronary intervention with bare metal stent vs. drug-eluting stent in hemodialysis patients. Circ J. 2012;76:1609-1615.
36. Ishio N, Kobayashi Y, Takebayashi H, et al. Impact of drug-eluting stents on clinical and angiographic outcomes in dialysis patients. Circ J. 2007;71:1525-1529.
37. Jeong YH, Hong MK, Lee CW, et al. Impact of significant chronic kidney disease on long-term clinical outcomes after drug-eluting stent versus bare metal stent implantation. Int J Cardiol. 2008;125:36-40.
38. Bae EH, Lim SY, Choi YH, et al. Drug-eluting vs. bare-metal stents for treatment of acute myocardial infarction with renal insufficiency. Results from Korea Acute Myocardial Infarction Registry. Circ J. 2011;75:2798-2804.
39. Kim BK, Oh S, Jeon DW, et al. Long-term clinical outcomes and stent thrombosis of sirolimus-eluting versus bare metal stents in patients with end-stage renal disease: results of Korean multicenter angioplasty team (KOMATE) registry. J Interv Cardiol. 2009;22:411-419.
40. Ahmed K, Jeong MH, Chakraborty R, et al. Coronary stents in patients with ST-elevation myocardial infarction and chronic kidney disease undergoing primary percutaneous coronary intervention. Korean Circ J. 2012;42:830-838.
41. Lemos PA, Arampatzis CA, Hoye A, et al. Impact of baseline renal function on mortality after percutaneous coronary intervention with sirolimus-eluting stents or bare metal stents. Am J Cardiol. 2005;95:167-172.
42. Charytan DM, Varma MR, Silbaugh TS, Lovett AF, Normand SL, Mauri L. Long-term clinical outcomes following drug-eluting or bare-metal stent placement in patients with severely reduced GFR: results of the Massachusetts Data Analysis Center (Mass-DAC) state registry. Am J Kidney Dis. 2011;57:202-211.
43. Meliga E, De Benedictis M, Gagnor A, et al. Clinical outcomes following percutaneous coronary intervention with drug-eluting stents versus bare metal stents in patients on chronic hemodialysis. J Interv Cardiol. 2013;26:351-358.
44. Moreyra AE, Hynes P, Deng Y, et al. Short and long-term outcomes of different revascularization strategies in patients with end stage renal disease on hemodialysis. J Am Coll Cardiol. 2012;59:E549.
45. Nakamura S, Nakamura S, Ogawa H, et al. Abstract 14215: Comparison of 4 years clinical outcome of stent implantation in renal failure patients with dialysis: comparison with bare metal stents, first and second generation drug-eluting stents. Circulation. 2012;126(21 Suppl):A14215.
46. Tsai TT, Messenger JC, Brennan JM, et al. Safety and efficacy of drug-eluting stents in older patients with chronic kidney disease: a report from the Linked CathPCI Registry–CMS Claims Database. J Am Coll Cardiol. 2011;58:1859-1869.
47. Green SM, Selzer F, Mulukutla SR, et al. Comparison of bare-metal and drug-eluting stents in patients with chronic kidney disease (from the NHLBI Dynamic Registry). Am J Cardiol. 2011;108:1658-1664.
48. Okada T, Hayashi Y, Toyofuku M, et al. One-year clinical outcomes of dialysis patients after implantation with sirolimus-eluting coronary stents. Circ J. 2008;72:1430-1435.
49. Crimi G, Leonardi S, Costa F, Adamo M, Ariotti S, Valgimigli M. Role of stent type and of duration of dual antiplatelet therapy in patients with chronic kidney disease undergoing percutaneous coronary interventions. Is bare metal stent implantation still a justifiable choice? A post-hoc analysis of the all comer PRODIGY trial. Int J Cardiol. 2016;212:110-117.
50. Resmini C, Di Cuia M, Ballocca F, et al. Short and long term outcome of percutaneous coronary intervention with drug eluting stent and bare metal stent in patients with chronic kidney disease. Minerva Cardioangiol. 2012;60:573-580.
51. Garg P, Charytan DM, Novack L, et al. Impact of moderate renal insufficiency on restenosis and adverse clinical events after sirolimus-eluting and bare metal stent implantation (from the SIRIUS trials). Am J Cardiol. 2010;106:1436-1442.
52. Suzuki K, Inoue N, Matsuo A, et al. [Limitation on efficacy of sirolimus-eluting stent implantation in patients on hemodialysis]. J Cardiol. 2007;49:331-336.
53. Simsek C, Magro M, Boersma E, et al. Impact of renal insufficiency on safety and efficacy of drug-eluting stents compared to bare-metal stents at 6 years. Catheter Cardiovasc Interv. 2012;80:18-26.
54. Halkin A, Mehran R, Casey CW, et al. Impact of moderate renal insufficiency on restenosis and adverse clinical events after paclitaxel-eluting and bare metal stent implantation: results from the TAXUS-IV trial. Am Heart J. 2005;150:1163-1170.
55. Chang TI, Montez-Rath ME, Tsai TT, Hlatky MA, Winkelmayer WC. Drug-eluting versus bare-metal stents during PCI in patients with end-stage renal disease on dialysis. J Am Coll Cardiol. 2016;67:1459-1469.
56. Yachi S, Tanabe K, Tanimoto S, et al. Clinical and angiographic outcomes following percutaneous coronary intervention with sirolimus-eluting stents versus bare-metal stents in hemodialysis patients. Am J Kidney Dis. 2009;54:299-306.
From 1New York Presbyterian Queens, Flushing, New York; 2Cardiac Clinic of Kissimmee, Kissimmee, Florida; 3Coronary and Structural Heart Diseases Department, Institute of Cardiology, Warsaw, Poland; 4Albany Medical College, Albany Medical Center, Albany, New York; 5Novosibirsk Research Institute of Circulation Pathology Named After Academician E.N. Meshalkin, Novosibirsk, Russia; 6Medical University of Warsaw, Warsaw, Poland; 7Bakulev Scientific Center for Cardiovascular Surgery, Moscow, Russia; 8Fortis Escorts Heart Institute, New Delhi, India; and 9New York University School of Medicine, New York, New York.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Bangalore reports research grants, honoraria, speaker’s bureau membership, and advisory board participation in Abbott Vascular; grant support from NHLBI (HL117905).
Manuscript submitted April 3, 2017, provisional acceptance given April 17, 2017, final version accepted May 5, 2017.
Address for correspondence: Sripal Bangalore, MD, MHA, FACC, FSCAI, Director of Research, Cardiac Catheterization Laboratory, Director, Cardiovascular Outcomes Group, Cardiovascular Clinical Research Center, Associate Professor of Medicine, New York University School of Medicine, The Leon H. Charney Division of Cardiology, New York, NY 10016. Email: sripal.bangalore@gmail.com











