Distal Open Sesame and Hairpin Wire Techniques to Facilitate a Chronic Total Occlusion Intervention
ABSTRACT: We describe a novel distal “open sesame” and “hairpin wire” technique application in a right coronary artery (RCA) chronic total occlusion intervention. Antegrade wiring was successful in entering an acute marginal branch distal to the occlusion, but not the mid RCA. After predilation, antegrade flow was restored but the mid RCA could not be wired in spite of using multiple different guidewires. A “hairpin” was created in a polymer jacketed guidewire, advanced into the acute marginal branch, and withdrawn, allowing wiring of the mid RCA, which was successfully stented using a variety of guide support and lesion preparation techniques.
J INVASIVE CARDIOL 2012;24(3):E57-E59
____________________________________________
The “open sesame” technique was described by Saito in 2010 for chronic total occlusions (CTO) that have a hard proximal cap and a side branch ramifying at the proximal end of the lesion.1 Insertion of a stiff guidewire and/or balloon inflation in the side branch induced a geometrical shift of the hard plaque and enabled guidewire entry into the CTO.1
Kawasaki et al first described the reversed guidewire technique in 2008.2 A hairpin was created at the distal end of a polymer jacketed guidewire and upon withdrawal, it crossed a highly angulated bifurcation lesion.2
We present a challenging coronary CTO percutaneous coronary intervention (PCI) case in which application of the “distal open sesame” and “hairpin wire” techniques enabled successful crossing and treatment of the occlusion.
 Case Report. A 63-year-old male patient with a history of PCI of the left anterior descending artery (LAD) 6 years prior, presented with recurrent stable angina that had persisted for more than a year despite optimal management. He had to use sublingual nitroglycerin 2 to 3 times a day for angina induced by mild exertion. Diagnostic angiography showed a CTO lesion at the mid right coronary artery (RCA) with patent LAD and circumflex arteries. As the patient was scheduled for transurethral resection of the prostate for benign prostatic hypertrophy, his CTO PCI was postponed for a few months until after prostatectomy, during which time he continued to experience daily exertional angina. After prostatectomy was successfully completed he was referred for elective PCI of the RCA CTO.
Case Report. A 63-year-old male patient with a history of PCI of the left anterior descending artery (LAD) 6 years prior, presented with recurrent stable angina that had persisted for more than a year despite optimal management. He had to use sublingual nitroglycerin 2 to 3 times a day for angina induced by mild exertion. Diagnostic angiography showed a CTO lesion at the mid right coronary artery (RCA) with patent LAD and circumflex arteries. As the patient was scheduled for transurethral resection of the prostate for benign prostatic hypertrophy, his CTO PCI was postponed for a few months until after prostatectomy, during which time he continued to experience daily exertional angina. After prostatectomy was successfully completed he was referred for elective PCI of the RCA CTO.
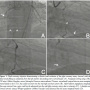
Bilateral femoral arterial access was obtained using 8 Fr 45 cm long sheaths. The RCA was initially engaged with an 8 Fr JR4 guide that provided poor to moderate support. The left main was engaged with a XB 3.5 guide. Coronary angiography using dual injection demonstrated marked calcification in the RCA with a 30 mm mid level occlusion (Figure 1A). The mid RCA filled via collaterals from the LAD and the circumflex and reconstitution of the mid RCA occurred at the site of origin of a large acute marginal branch.
We initially attempted to cross antegradely using several wires including the Fielder XT and Confianza Pro 12 wire (Asahi Intecc) through a 1.5 mm x 8 mm over-the-wire balloon without success. The JR4 guide did not provide enough support and was changed for an AL1 guide that did not fit well and provided marginally better support. We tried to cross with a CrossBoss catheter (Bridgepoint Medical) unsuccessfully, followed by a Finecross (Terumo) with Pilot 200 (Abbott Vascular) and Confianza Pro 12 (Asahi Intecc) wires, also unsuccessfully. We changed the guide to an AL 0.75 but were still unable to cross with various wires including Pilot 200, Progress 200, and Pilot 150 (Abbott Vascular).
We attempted to wire retrogradely, but were unable to cross through septal collaterals in part due to a previously placed LAD stent. We engaged the RCA with a 7 Fr Champ 2 guide and wired into the acute marginal distal to the CTO using a Pilot 150 wire (Abbott Vascular) through a Finecross microcatheter (Terumo) (Figure 1B). We encountered severe difficulty crossing the CTO with a balloon. We inflated several 1.5 mm balloons into the lesion but were unsuccessful in crossing. We used a 7 Fr Guideliner catheter (Vascular Solutions) to deeply engage the RCA and were then able to cross with a 1.5 mm balloon. We predilated several times with 1.5 mm and 2.0 mm balloons, restoring antegrade flow through the RCA CTO (Figure 1C).
We could not wire antegradely into the mid RCA in spite of using multiple wires, as there was an acute bend at the origin of the acute marginal branch. We eventually used a "hairpin wire" technique, in which a Whisper wire (Abbott Vascular) was bent approximately 3 cm from its tip, advanced into the acute marginal branch, and pulled back (Figures 1D and 1E). We then attempted to advance a balloon into the distal RCA, but were not successful and in the process, the guidewire and guide catheter position were lost.
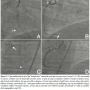 We engaged the RCA again with an 8 Fr JR4 guide and rewired into the acute marginal branch distal to the CTO and into the distal RCA using the same "hairpin wire" technique. We once again encountered significant difficulty delivering equipment to the distal RCA. Several 1.5 mm balloons could not cross. We were finally able to advance a Corsair catheter (Asahi Intecc) (arrow, Figure 2B) to the distal RCA using an anchor balloon technique with a 2.5 mm balloon into a small proximal RCA conus branch (arrowhead, Figure 2B). The Whisper wire (Abbott Vascular) was exchanged for an Ironman wire (Abbott Vascular, arrowhead, Figure 2C), which enabled sequential delivery of 2.0, 2.5, and 3.0 mm balloons and predilation of the entire proximal, mid, and distal RCA. We were then able to deliver three 3.0 mm x 28 mm Xience V everolimus-eluting stents (Abbott Vascular) using a Guideliner catheter (Vascular Solutions, arrow, Figure 2C) for extra guide support. The stents were postdilated with a 3.5 mm non-compliant balloon, providing an excellent final angiographic result (Figure 2D), as confirmed by intravascular ultrasonography. The total fluoroscopy time and air kerma radiation dose were 39.2 minutes and 7.4 Gray, respectively, and 358 mL of contrast were administered. At 1-month follow-up the patient was angina-free and had significantly increased his daily activities.
We engaged the RCA again with an 8 Fr JR4 guide and rewired into the acute marginal branch distal to the CTO and into the distal RCA using the same "hairpin wire" technique. We once again encountered significant difficulty delivering equipment to the distal RCA. Several 1.5 mm balloons could not cross. We were finally able to advance a Corsair catheter (Asahi Intecc) (arrow, Figure 2B) to the distal RCA using an anchor balloon technique with a 2.5 mm balloon into a small proximal RCA conus branch (arrowhead, Figure 2B). The Whisper wire (Abbott Vascular) was exchanged for an Ironman wire (Abbott Vascular, arrowhead, Figure 2C), which enabled sequential delivery of 2.0, 2.5, and 3.0 mm balloons and predilation of the entire proximal, mid, and distal RCA. We were then able to deliver three 3.0 mm x 28 mm Xience V everolimus-eluting stents (Abbott Vascular) using a Guideliner catheter (Vascular Solutions, arrow, Figure 2C) for extra guide support. The stents were postdilated with a 3.5 mm non-compliant balloon, providing an excellent final angiographic result (Figure 2D), as confirmed by intravascular ultrasonography. The total fluoroscopy time and air kerma radiation dose were 39.2 minutes and 7.4 Gray, respectively, and 358 mL of contrast were administered. At 1-month follow-up the patient was angina-free and had significantly increased his daily activities.
Discussion. Our report demonstrates the following: (1) wiring into a side branch at the CTO distal cap and subsequently performing balloon angioplasty into the side branch can restore antegrade flow into the main vessel (“distal open-sesame technique”); (2) the “hairpin wire” technique can be useful in directing a guidewire from the side branch to the main vessel; and (3) use of multiple guide support enhancement techniques and of the Corsair microcatheter (Abbott Vascular) may enable successful lesion crossing, once conventional strategies fail.
The “open sesame” technique was first described by Saito in 4 challenging CTOs.1 The author demonstrated how insertion of stiff wires and balloon inflation of a side branch at the CTO proximal cap facilitated antegrade CTO wiring, likely by inducing a geographic shift of the atherosclerotic plaque. In our case we describe a modification of the open sesame technique, the “distal open sesame technique,” in which a wire is advanced into a side branch at the distal CTO cap. After balloon angioplasty into the side branch, antegrade flow was restored.
After multiple guidewires failed to enter the main vessel distal to the CTO, the "hairpin wire" technique was utilized successfully. A polymer-jacketed wire was bent approximately 3 cm from the wire tip, advanced to the acute marginal branch, and pulled back entering the main vessel. This technique was first described by Kawasaki et al to access an extremely angulated bifurcation, and was given the name “reversed guidewire’’ technique.2 A modification of this technique named “hairpin-trap” technique was recently described for stent retrieval.3 In the current case, the hairpin wire technique was useful in directing a guidewire through a highly angulated acute marginal branch into the mid RCA.
Finally, multiple techniques were applied throughout the case to facilitate equipment delivery through the lesion. The Guideliner catheter (Vascular Solutions),4 the side branch anchor technique,5 and a stiff coronary guidewire (Ironman, Abbott Vascular) were used to increase guide catheter support. The Corsair microcatheter (Asahi Intecc) was used to create a channel through the lesion when balloon crossing failed. Although the Tornus catheter (Asahi Intecc) is most commonly used in “balloon uncrossable” CTOs, our case demonstrates that the Corsair catheter can also be useful in such cases, especially in combination with guide support enhancing techniques.6 The Corsair catheter (Asahi Intecc) was originally developed as a collateral channel dilator to facilitate retrograde CTO PCI. Tsuchikane et al reported successful channel crossing in 90 of 93 (96.8%) CTO lesions.7 Similarly, Rinfret et al reported use of Corsair microcatheter in 38 (90%) of 42 consecutive cases who underwent retrograde recanalization of CTO using transradial approach.8 Our case report extents the use of the Corsair for “balloon uncrossable” CTOs, but also highlights the importance of using multiple combinations of techniques to achieve a successful outcome.6
The above-described techniques have important limitations. To perform the distal “open sesame” technique, antegrade crossing into a side branch at the distal cap is required, which may not be feasible. Moreover, balloon angioplasty into the side branch may cause plaque shift and occlusion of the distal main vessel. Moreover, wiring into the main branch distal to the CTO may be challenging due to angulation, as in our case, requiring use of additional wiring techniques, such as the “hairpin wire” technique. Use of the latter technique should be done with caution, as it may cause vessel dissection and because after entering the main vessel with the wire tip, further advancement may be challenging due to the bent wire. Use of the Corsair catheter (Asahi Intecc) or any other technique for crossing a CTO should only be performed after dual coronary injection has confirmed that the guidewire is located within the distal true lumen.
Conclusion. In summary, the distal “open sesame” and the “hairpin wire” techniques can be useful tools for interventionalists performing challenging CTO interventions.
Acknowledgment. We gratefully acknowledge the tremendous support of the cardiac catheterization laboratory team at the Dallas VA Medical Center for enabling the development of novel catheterization techniques and the performance of clinical research.
References
- Saito S. Open Sesame Technique for chronic total occlusion. Catheter Cardiovasc Interv. 2010 Apr;75(5):690-694.
- Kawasaki T, Koga H, Serikawa T. New bifurcation guidewire technique: a reversed guidewire technique for extremely angulated bifurcation--a case report. Catheter Cardiovasc Interv. 2008 Jan;71(1):73-76.
- Brilakis ES, Abdel-Karim AR, Banerjee S. Hairpin-trap: a novel stent retrieval technique. Catheter Cardiovasc Interv. 2011 Feb;77(2):213-216.
- Luna M, Papayannis A, Holper EM, Banerjee S, Brilakis ES. Transfemoral use of the Guideliner catheter in complex coronary and bypass graft interventions. Cathet Cardiovasc Interv. 2011 Jul:[Epub ahead of print].
- Fujita S, Tamai H, Kyo E, et al. New technique for superior guiding catheter support during advancement of a balloon in coronary angioplasty: the anchor technique. Catheter Cardiovasc Interv. 2003 Aug;59(4):482-488.
- Brilakis ES, Banerjee S. Crossing the “balloon uncrossable” chronic total occlusion: Tornus to the rescue. Cathet Cardiovasc Interv. 2011 Sep;78(3):363-365.
- Tsuchikane E, Katoh O, Kimura M, Nasu K, Kinoshita Y, Suzuki T. The first clinical experience with a novel catheter for collateral channel tracking in retrograde approach for chronic coronary total occlusions. JACC Cardiovasc Interv. 2010 Feb;3(2):165-171.
- Rinfret S, Joyal D, Nguyen CM, et al. Retrograde recanalization of chronic total occlusions from the transradial approach; early Canadian experience. Catheter Cardiovasc Interv. 2011 Sep;78(3):366-374.
____________________________________________
From the VA North Texas Healthcare System and University of Texas Southwestern Medical Center at Dallas, Dallas, Texas.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Michael is supported by the T32HL007360 Cardiovascular Training Grant from the National Institutes of Health. Dr. Banerjee reports speaker honoraria from St. Jude Medical, Medtronic, and Johnson & Johnson and research support from Boston Scientific and The Medicines Company. Dr. Brilakis reports speaker honoraria from St Jude Medical and Terumo; research support from Abbott Vascular; salary from Medtronic (spouse).
Manuscript submitted September 9, 2011, provisional acceptance given October 3, 2011, final version accepted October 6, 2011.
Address for correspondence: Emmanouil S. Brilakis, MD, PhD, VA North Texas Health Care System, The University of Texas Southwestern Medical Center at Dallas, Division of Cardiology (111A), 4500 S. Lancaster Rd., Dallas, TX, 75216, USA. Email: esbrilakis@yahoo.com












