Differences in Optical Coherence Tomographic Findings and Clinical Outcomes Between Excimer Laser and Cutting Balloon Angioplasty for Focal In-Stent Restenosis Lesions
Abstract: Aim. In-stent restenosis (ISR), especially focal ISR, after percutaneous coronary intervention (PCI) remains one of the major clinical problems in the drug-eluting stent (DES) era. Several reports have revealed that excimer laser coronary angioplasty (ELCA) is useful for ISR; however, detailed findings after ELCA are unknown. Therefore, we investigated the condition of the neointima after ELCA for ISR with optical coherence tomography (OCT) and compared the OCT findings and clinical outcome between ELCA and cutting-balloon angioplasty (CBA). Methods. Twenty-one consecutive patients with focal ISR who underwent ELCA or CBA were enrolled. All patients underwent 12- to 15-month follow-up coronary angiography. OCT was performed immediately after successful PCI to evaluate the neointimal condition in the ISR lesion. We compared the following OCT parameters between ELCA and CBA groups: maximal thickness of remaining in-stent neointima (MTN), number of tears, minimum lumen dimension (MLD), and minimum lumen area (MLA). We also evaluated clinical outcomes, including target vessel revascularization, acute myocardial infarction, death, and stent thrombosis. Results. MLA in the ELCA group (n = 10) was significantly larger than in the CBA group, and number of tears in the ELCA group was significantly lower than in the CBA group. A trend was shown toward lower TLR with ELCA versus CBA (10.0% vs 45.5%). Conclusions. OCT immediately after ELCA for ISR lesions revealed larger lumen area and smaller number of tears compared with CBA, which may support favorable effects of ELCA for focal ISR.
J INVASIVE CARDIOL 2012;24(10):478-483
Key words: optical coherence tomography, excimer laser, cutting-balloon angioplasty
________________________________________________________________
The drug-eluting stent (DES) can markedly reduce restenosis after coronary intervention.1,2 However, in-stent restenosis (ISR) is still a major troublesome problem even in the DES era because optimal treatment has not been established for ISR. Some reports have suggested that cutting-balloon angioplasty (CBA) may be a favorable method for treating ISR, especially focal ISR,3-6 but another report did not support a beneficial effect of CBA for ISR.7 Recently, excimer-laser coronary angioplasty (ELCA) has been applied to various coronary and endovascular intervention therapies. Although it is used to treat peripheral artery disease and the available clinical data support ELCA as a reliable technology for endovascular intervention,8-10 in the field of coronary artery intervention, the effects of ELCA remain controversial.11-14 Certainly, repeat DES is one useful retreatment modality for DES restenosis,15,16 but a second layer of metal is not desirable in the coronary artery due to its effects on distensibility of the artery and the difficulty imposed on future treatment. Accordingly, ELCA and CBA have potential efficacy for the treatment of focal ISR, but the detailed mechanism of this efficacy has not yet been investigated.
Optical coherence tomography (OCT) is a new imaging modality that visualizes intracoronary features with an axial resolution of 3-20 μm, which is substantially higher than with intravascular ultrasound (IVUS) (100-150 μm). In the present study, we used OCT to evaluate lumen condition in detail immediately after ELCA or CBA for focal ISR and compared the efficacy of ELCA and CBA.
Methods
Between October 2008 and August 2009, consecutive patients who underwent ELCA or CBA for focal ISR after DES implantation were enrolled in the present study. ELCA or CBA were selected at random. Angiographic ISR was defined as an in-stent diameter stenosis of >50% by quantitative coronary angiography (QCA) (QAngio XA, Version 7.1; MEDIS Medical Imaging Systems). Focal ISR was defined as an ISR lesion of <10 mm in length according to Mehran’s classification.17 We excluded patients with diffuse ISR,17 and for these patients, repeat DES implantation was performed. The other exclusion criteria included contraindication to antiplatelet therapy, renal dysfunction (serum creatinine >2.5 mg/dL) without hemodialysis, a history of prior myocardial infarction, and cardiogenic shock. ELCA or CBA were performed if the patient satisfied the criteria proposed by the Academic Research Consortium.18 All study patients showed symptomatic or documented ischemia before the procedures. The original stents, which had been implanted by IVUS-guided interventional procedures in all study patients, were either sirolimus-eluting stents (Cypher, Cordis Corporation) or paclitaxel-eluting stents (Taxus Express2, Boston Scientific). Follow-up coronary angiography was scheduled at 1 year after ELCA or CBA in all patients. In our hospital, all patients with DES implantation received dual antiplatelet therapy with aspirin 100 mg/day and clopidogrel 75 mg/day until follow-up coronary angiography. The medical ethics committee at our hospital approved this study, and written informed consent was obtained from all patients before each catheterization.
Coronary intervention procedures were performed via radial approach using a 6 Fr sheath or a femoral approach using a 7 Fr sheath. Heparin (5000 IU) was intravenously administered and 2.5 mg isosorbide dinitrate was administered into the affected coronary artery via the coronary angiographic catheter. An activated clotting time of >300 seconds was maintained during ELCA or CBA procedures. After a 0.014˝ guidewire crossed the stenotic lesion, ELCA was performed with a Spectranetics CVS-300 System (Spectranetics), which is a XeCl excimer-laser system. The laser catheters used were Vitesse E eccentric multifiber catheters (Spectranetics) with diameters of 1.4-2.0 mm. The selection of catheter size depended on the target lesion and vessel, and a smaller catheter was favored intentionally (1.7 mm for 2.5-3 mm stents and 2.0 mm for 3.5 mm stents). If the optimal laser catheter could not pass the lesion, a 1.4 mm catheter was passed first. The laser catheter was advanced at a speed of 0.5 mm/sec across the lesion, with standard saline flush technique applied.19 We used the area ablation method and debulked the lesion step-by-step. Only 5 mm of the lesion were debulked during each pass. The catheter was pulled back and rotated clockwise by 90 degrees and re-advanced 5 mm. We repeated the procedure by rotating the laser catheter clockwise to 180 and 270 degrees. Energy densities ranged from 35 to 55 mJ/mm2 (mean, 39.5 ± 5.5 mJ/mm2), and the pulse number was 2500. The multiple laser pass technique was used in all lesions. Ablation time ranged from 25 to 50 seconds. After debulking with ELCA, adjunctive balloon angioplasty at a low inflation pressure (3.4 ± 0.5 atm) was performed. Procedure success was defined as final diameter stenosis of <25% visually.
In the CBA procedures, after a 0.014˝ guidewire crossed the stenotic lesion, a cutting balloon (Flextome Cutting Balloon, Boston Scientific) was used. Multiple inflations (4-6 inflations, each at 8 atm) were allowed for focal ISR lesions until an optimal final result, which was defined as a residual (luminal) diameter stenosis of <25% visually, was obtained.
We used the same-size balloon as the implanted stent when we performed CBA and post-ELCA balloon angioplasty.
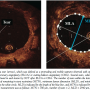 OCT was performed in all patients immediately after the ELCA or CBA procedure. OCT acquisition was performed with a commercially available system (M3 OCT system, Light Lab Imaging). The image wire (ImageWire, LightLab Imaging) was positioned distal to the region of interest with an over-the-wire OCT catheter (Helios OBC, LightLab Imaging) that had been placed in the artery over a conventional guidewire. Then, the OCT catheter was withdrawn from the distal to proximal end of the stent. Automated pullback was performed at 1.5 mm/s while blood was removed by continuous infusion of Lactated Ringer’s solution at 0.5-1.5 mL/s using a power injector (Mark V ProVis Injection Systems, Integrated Pedestal Configuration Medrad Inc). Using OCT, we assessed the maximal thickness of remaining in-stent neointima (MTN), the number of tears, minimum lumen dimension (MLD), and minimum lumen area (MLA) (Figure 1). Two independent observers who were blinded to the patient’s clinical background and angiographic lesion characteristics performed offline analysis of the OCT images.
OCT was performed in all patients immediately after the ELCA or CBA procedure. OCT acquisition was performed with a commercially available system (M3 OCT system, Light Lab Imaging). The image wire (ImageWire, LightLab Imaging) was positioned distal to the region of interest with an over-the-wire OCT catheter (Helios OBC, LightLab Imaging) that had been placed in the artery over a conventional guidewire. Then, the OCT catheter was withdrawn from the distal to proximal end of the stent. Automated pullback was performed at 1.5 mm/s while blood was removed by continuous infusion of Lactated Ringer’s solution at 0.5-1.5 mL/s using a power injector (Mark V ProVis Injection Systems, Integrated Pedestal Configuration Medrad Inc). Using OCT, we assessed the maximal thickness of remaining in-stent neointima (MTN), the number of tears, minimum lumen dimension (MLD), and minimum lumen area (MLA) (Figure 1). Two independent observers who were blinded to the patient’s clinical background and angiographic lesion characteristics performed offline analysis of the OCT images.
Coronary angiography was performed in all study patients immediately after and at 1 year after ELCA or CBA. All angiography was performed using 6 Fr or 7 Fr catheters. QCA was performed with an automated detection algorithm with the contrast-filled catheter as the calibration standard. Minimal lumen diameter, reference stent diameter, diameter stenosis, and lesion length were measured from multiple projections, and results from the “worst” view were recorded. Late loss was calculated by subtraction of minimal lumen diameter at follow-up coronary angiography from minimal lumen diameter measured immediately after the procedure.
We compared the following traditional coronary risk factors, medications, interventions, and serum parameters between the ELCA and CBA groups. Coronary risk factors evaluated included incidence of dyslipidemia, hypertension, diabetes mellitus, and smoking. Dyslipidemia was defined as patients treated with medication or whose serum low-density lipoprotein cholesterol level was ≥140 mg/dL; hypertension was defined as patients whose blood pressure was ≥150 mm Hg despite therapy for at least 3 months; and diabetes mellitus was defined as patients with type 2 diabetes currently treated with hypoglycemic agents or having a history of diabetes.20 Smoking was classified as smoker or non-smoker. We evaluated the following prescribed medications: statins, anticoagulants, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, calcium antagonists, and β-blockers. Administration of these medications began just after stenting and was not changed during the follow-up period in any of the patients. As interventional parameters, we evaluated lesion type (American College of Cardiology/American Heart Association type A/B1 or B2/C);21 stent length and diameter, which were determined by IVUS (Atlantis Pro; Boston Scientific) findings during the original interventional procedure; the maximal inflation pressure for stent implantation; and the interval between the original stent implantation and the interventional procedure for ISR.
We compared clinical outcomes, including target lesion revascularization (TLR), acute myocardial infarction, cardiac death, and stent thrombosis until the follow-up coronary angiography, between ELCA and CBA groups. If the patients showed restenosis (% diameter stenosis >50%) at the follow-up coronary angiography without typical chest pain, we evaluated ischemia using exercise Tl-201 single-photon emission computed tomography (SPECT) before the reintervention. All patients with restenosis who complained of typical chest pain or showed ischemia by SPECT were selected to undergo re-intervention according to the report by Cutlip et al.18
Data are expressed as mean ± standard deviation. The chi-square test was used to assess differences in categorical variables between the two groups, and the Student’s t-test was used to assess differences in continuous variables between the two groups. To evaluate the reproducibility of OCT parameters including MTN, number of tears, MLD, and MLA, inter-rater reliability was evaluated by Cohen’s kappa coefficient, with values >0.80 being considered to show almost perfect agreement. A value of P<.05 was considered statistically significant. Statistical analysis was performed with Stat View 5.0 MDSU statistical software (SAS Institute).
Results
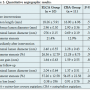
 During the study period, we treated 10 patients with focal ISR by ELCA (8 men and 2 women; age, 69.5 ± 9.4 years) and 11 patients by CBA (8 men and 3 women; age, 65.7 ± 5.5 years). Baseline characteristics of the two groups are summarized in Table 1. Rates of sirolimus-eluting stent implantation, coronary risk factors, medication use, and interventional factors were similar between the ELCA and CBA groups.
During the study period, we treated 10 patients with focal ISR by ELCA (8 men and 2 women; age, 69.5 ± 9.4 years) and 11 patients by CBA (8 men and 3 women; age, 65.7 ± 5.5 years). Baseline characteristics of the two groups are summarized in Table 1. Rates of sirolimus-eluting stent implantation, coronary risk factors, medication use, and interventional factors were similar between the ELCA and CBA groups.
 All procedures were successful without major complications including death, myocardial infarction, and perforation. QCA analysis revealed no significant differences in baseline angiographic measurements between the two groups. Immediately after intervention, there were no significant differences in QCA data including acute gain between the two groups, but 1-year follow-up QCA data showed that significantly lower late loss and diameter stenosis and a trend toward greater MLD were achieved in the ELCA group as compared with the CBA group (Table 2).
All procedures were successful without major complications including death, myocardial infarction, and perforation. QCA analysis revealed no significant differences in baseline angiographic measurements between the two groups. Immediately after intervention, there were no significant differences in QCA data including acute gain between the two groups, but 1-year follow-up QCA data showed that significantly lower late loss and diameter stenosis and a trend toward greater MLD were achieved in the ELCA group as compared with the CBA group (Table 2).
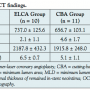 OCT was successfully performed immediately after ELCA or CBA in all patients. In this study, each patient who underwent ELCA received adjunctive balloon angioplasty. MLA in the ELCA group was significantly larger than in the CBA group, whereas the number of tears in the ELCA group was significantly lower than in the CBA group (Table 3). MTN and MLD showed a tendency toward a higher value in the ELCA group than in the CBA group. Coronary angiography after ELCA or CBA showed no dissection or irregular lesion in any of the study patients. Intra-observer variability of MTN, MLD, and MLA showed Cohen’s kappa coefficients of 0.935, 0.926, and 0.961, respectively. Intra-observer variability of tears showed a Cohen’s kappa coefficient of 1.000. However, inter-observer variability of MTN, MLD, and MLA showed Cohen’s kappa coefficients of 0.932, 0.894, and 0.943, respectively. Inter-observer variability of tears also showed a Cohen’s kappa coefficient of 1.000.
OCT was successfully performed immediately after ELCA or CBA in all patients. In this study, each patient who underwent ELCA received adjunctive balloon angioplasty. MLA in the ELCA group was significantly larger than in the CBA group, whereas the number of tears in the ELCA group was significantly lower than in the CBA group (Table 3). MTN and MLD showed a tendency toward a higher value in the ELCA group than in the CBA group. Coronary angiography after ELCA or CBA showed no dissection or irregular lesion in any of the study patients. Intra-observer variability of MTN, MLD, and MLA showed Cohen’s kappa coefficients of 0.935, 0.926, and 0.961, respectively. Intra-observer variability of tears showed a Cohen’s kappa coefficient of 1.000. However, inter-observer variability of MTN, MLD, and MLA showed Cohen’s kappa coefficients of 0.932, 0.894, and 0.943, respectively. Inter-observer variability of tears also showed a Cohen’s kappa coefficient of 1.000.
The results of QCA data at 1-year follow-up coronary angiography showed restenosis (>50% diameter stenosis) to be present in 2 patients (20%) in the ELCA group and 5 patients in the CBA group (45.5%). Among these patients, 1 required TLR (10%) in the ELCA group and 5 required TLR (45.5%) in the CBA group. Thus, there was a trend toward less frequent need for 1-year TLR in the ELCA group versus the CBA group. No cardiac deaths, myocardial infarctions, or stent thromboses occurred during the follow-up period.
Discussion
ven in the DES era, a low rate of ISR still exists after DES implantation; moreover, we cannot neglect the prevalence of ISR because the population treated with DES is very large. The predominant morphologic pattern of restenosis in bare-metal stents (BMSs) is the diffuse type, but in DESs is the focal type.6,22,23 If the ISR lesion is diffuse, repeat implantation of a DES is favorable, but if the morphologic type is focal, implantation of a second layer of metal by repeat DES is not recommended.22 Moreover, a recent study showed no significant difference in TLR rates at 2 years after intervention for ISR between repeat DES and balloon angioplasty.16 However, balloon angioplasty for treatment of ISR resulted in a high rate of recurrent restenosis of >50%.24-27 Several reports have shown ELCA and CBA to be effective for ISR;3-6,26 therefore, in the present study, we focused on the treatment of focal ISR by ELCA and CBA, and we evaluated detailed findings with OCT immediately after interventional therapy. Our results indicated that ELCA tended to produce better long-term results than CBA for focal ISR lesions after DES implantation due to a larger MLA and less vascular injury as demonstrated by OCT.
The mechanism of ELCA in lumen enlargement in non-stented lesions is a combination of tissue ablation and vessel expansion. However, in ISR lesions, vessel expansion is limited by the stent; thus, the entire lumen enlargement gained after ELCA in ISR lesions is the result of neointimal tissue ablation.26,28 Mehran et al26 reported that adjunctive balloon angioplasty just after ELCA with an inflation pressure of 14.5 ± 4.4 atm was responsible for 71% of overall lumen gain. Additional stent expansion after adjunctive balloon angioplasty is certainly important to attain larger lumen gain; however, it can produce more vascular injury, which may induce additional neointimal hyperplasia in the chronic phase. In fact, it has been reported that the highest acute gain and the largest acute MLD were obtained after stent implantation for an ISR lesion versus after rotablator and balloon angioplasty, but these acute results were offset by a higher late lumen loss (almost 47%) at follow-up.29 Therefore, in the present study, we used adjunctive balloon angioplasty with mild inflation pressures of 3.4 ± 0.5 atm to reduce vascular injury. As a result, a favorable TLR rate of 10% was achieved at 1 year after the procedure. Thus, we believe it is better to perform adjunctive balloon angioplasty with mild inflation pressures after ELCA.
The mechanism of lumen enlargement after CBA depends on the presence of microblades that incise the atherosclerotic plaque or neointimal tissue at the beginning of balloon inflation and the development of controlled fault lines along which dilation will occur. Thus, in the ISR lesion, CBA can achieve plaque extrusion through the stent’s struts with less tissue injury.30 Another favorable effect of CBA in ISR lesions is avoidance of balloon slippage in the ISR lesion during the procedure,6 which is a common technical problem of balloon angioplasty in ISR lesions during inflation.
ISR is largely attributed to neointimal hyperplasia.31 Therefore, ideal therapy for ISR requires tissue ablation and vessel expansion with less injury, which leads to lower upregulation of Mac-1 and consequently preserves lumen area and lowers the restenosis rate.32 Even if higher acute gain and larger acute MLD are obtained after severe vascular injury, these acute results are offset by a high late lumen loss at follow-up.29,33 Because the resolution of OCT (3-20 μm) is substantially higher than that of IVUS (100-150 μm) and coronary angiography, OCT in the present study revealed less vascular injury, which was determined by the smaller number of tears, after ELCA with adjunctive balloon angioplasty than after CBA, whereas tears could not be detected in either group with coronary angiography. OCT also revealed larger MLA in the ELCA group than in the CBA group, whereas the minimal lumen diameter values by QCA were similar between the two groups. One of the most important factors in predicting TLR is final procedural luminal gain.26 Therefore, we believe that larger acute gain with less vascular injury seen with ELCA versus CBA, which was documented by OCT, resulted in the better clinical results achieved by ELCA in this study, even if QCA results were similar between the two groups. On the cost differential between ELCA and CBA, the former is slightly expensive. However, the reduced number of procedures for ISR using ELCA as compared to CBA may contribute to cost-reduction from the long-term perspective.
A previous report showed the long-term results of ELCA with adjunctive balloon angioplasty to be superior to balloon angioplasty alone.26 However, it has been reported that the TLR rates of ELCA with adjunctive balloon angioplasty and CBA were similar at approximately 30%.29,34 In our study, the TLR rate after ELCA for focal ISR showed a tendency toward better results than after CBA. In addition, the TLR rate of the ELCA group in this study was better than in previous reports26,34 while the TLR rate of CBA in this study was similar to other previous reports on CBA.29,35 The reason for the better TLR rate in the ELCA group in this study may be partially explained by the lower inflation pressures used during adjunctive balloon angioplasty than those reported previously,34 which might induce less vascular injury that results in better chronic clinical outcomes.
There were some reports of earlier rotablator trials where subsequent low pressure inflations did not improve outcomes.34,36-38 However, the mechanism of ablation using ELCA and rotablator are different. The former is photoablation of the tissue and the latter is mechanical grating of the tissue. Moreover, these reports using rotablator were published in the BMS era and so the major ISR pattern showed diffuse ISR.34,36-38 In addition, it has been reported that histological findings of ISR in DES is different from those in BMS.39,40 Therefore, we believe ELCA with adjunctive balloon angioplasty is favorable in subsets of focal ISR of DES.
To the best of our knowledge, this is the first report to describe a larger MLA with less vascular injury detected by OCT after ELCA than after CBA for the treatment of focal ISR. Although the number of study patients was small, this was a randomized and prospective study, and, thereby, these data are valuable in the DES era. The data suggest that ELCA with adjunctive balloon angioplasty performed with mild inflation pressures may be one recommended strategy for focal ISR. To confirm our results, a larger prospective and randomized study should be performed.
References
- Dibra A, Kastrati A, Alfonso F, et al. Effectiveness of drug-eluting stents in patients with bare-metal in-stent restenosis: meta-analysis of randomized trials. J Am Coll Cardiol. 2007;49(5):616-623.
- Kastrati A, Dibra A, Spaulding C, et al. Meta-analysis of randomized trials on drug-eluting stents vs. bare-metal stents in patients with acute myocardial infarction. Eur Heart J. 2007;28(22):2706-13.
- Miyamoto T, Araki T, Hiroe M, et al. Standalone cutting balloon angioplasty for the treatment of stent-related restenosis: acute results and 3- to 6-month angiographic recurrent restenosis rates. Catheter Cardiovasc Interv. 2001;54(3):301-308.
- Albiero R, Silber S, Di Mario C, et al. Cutting balloon versus conventional balloon angioplasty for the treatment of in-stent restenosis: results of the restenosis cutting balloon evaluation trial (RESCUT). J Am Coll Cardiol. 2004;43(6):943-949.
- Cin VG, Pekdemir H, Akkus MN, et al. Cutting balloon angioplasty for the treatment of in-stent restenosis in diabetics: A matched comparison of 6 months’ outcome with conventional balloon angioplasty. Angiology. 2006;57(4):445-452.
- Dangas GD, Claessen BE, Caixeta A, et al. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56(23):1897-1907.
- Park SJ, Kim KH, Oh IY, et al. Comparison of plain balloon and cutting balloon angioplasty for the treatment of restenosis with drug-eluting stents vs bare metal stents. Circ J. 2010;74(9):1837-1845.
- Tan JW, Yeo KK, Laird JR. Excimer laser-assisted angioplasty for infrainguinal artery disease. J Cardiovasc Surg. 2008;49(3):329-340.
- Dave RM, Patlola R, Kollmeyer K, et al. Excimer laser recanalization of femoropopliteal lesions and 1-year patency: results of the CELLO registry. J Endovasc Ther. 2009;16(6):665-675.
- Micari A, Vadala G, Biamino G. Update on the TURBO BOOSTER spectranetics laser for lower extremity occlusive disease. J Cardiovasc Surg. 2010;51(2):233-243.
- Hamburger JN, Foley DP, de Feyter PJ, et al. Six-month outcome after excimer laser coronary angioplasty for diffuse in-stent restenosis in native coronary arteries. Am J Cardiol. 2000;86(4):390-394.
- Dahm JB, Kuon E. High-energy eccentric excimer laser angioplasty for debulking diffuse iIn-stent restenosis leads to better acute- and 6-month follow-up results. J Invasive Cardiol. 2000;12(7):335-342.
- Koster R, Kahler J, Terres W, et al. Six-month clinical and angiographic outcome after successful excimer laser angioplasty for in-stent restenosis. J Am Coll Cardiol. 2000;36(1):69-74.
- Dahm JB. Excimer laser coronary angioplasty (ELCA) for diffuse in-stent restenosis: beneficial long-term results after sufficient debulking with a lesion-specific approach using various laser catheters. Lasers Med Sci. 2000;16(2):84-89.
- Alfonso F, Perez-Vizcayno MJ, Hernandez R, et al. A randomized comparison of sirolimus-eluting stent with balloon angioplasty in patients with in-stent restenosis: results of the Restenosis Intrastent: Balloon Angioplasty Versus Elective Sirolimus-Eluting Stenting (RIBS-II) trial. J Am Coll Cardiol. 2006;47(11):2152-2160.
- Tagliareni F, La Manna A, Saia F, et al. Long-term clinical follow-up of drug-eluting stent restenosis treatment: retrospective analysis from two high volume catheterisation laboratories. EuroIntervention. 2010;5(6):703-708.
- Mehran R, Dangas G, Abizaid AS, et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100(18):1872-1878.
- Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344-2351.
- Bittl JA, Sanborn TA. Excimer laser-facilitated coronary angioplasty. Relative risk analysis of acute and follow-up results in 200 patients. Circulation. 1992;86(1):71-80.
- Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. Jama. 2006;295(2):180-189.
- Schachinger V, Allert M, Kasper W, et al. Adjunctive intracoronary infusion of antithrombin III during percutaneous transluminal coronary angioplasty. Results of a prospective, randomized trial. Circulation. 1994;90(5):2258-2266.
- Corbett SJ, Cosgrave J, Melzi G, et al. Patterns of restenosis after drug-eluting stent implantation: Insights from a contemporary and comparative analysis of sirolimus- and paclitaxel-eluting stents. Eur Heart J. 2006;27(19):2330-2337.
- Colombo A, Orlic D, Stankovic G, et al. Preliminary observations regarding angiographic pattern of restenosis after rapamycin-eluting stent implantation. Circulation. 2003;107(17):2178-2180. Epub 2003 Apr 28.
- Gordon PC, Gibson CM, Cohen DJ, et al. Mechanisms of restenosis and redilation within coronary stents--quantitative angiographic assessment. J Am Coll Cardiol. 1993;21(5):1166-1174.
- Mehran R, Mintz GS, Popma JJ, et al. Mechanisms and results of balloon angioplasty for the treatment of in-stent restenosis. Am J Cardiol. 1996;78(6):618-622.
- Mehran R, Mintz GS, Satler LF, et al. Treatment of in-stent restenosis with excimer laser coronary angioplasty: mechanisms and results compared with PTCA alone. Circulation. 1997;96(7):2183-2189.
- Eltchaninoff H, Koning R, Tron C, et al. Balloon angioplasty for the treatment of coronary in-stent restenosis: immediate results and 6-month angiographic recurrent restenosis rate. J Am Coll Cardiol. 1998;32(4):980-984.
- Mintz GS, Kovach JA, Javier SP, et al. Mechanisms of lumen enlargement after excimer laser coronary angioplasty. An intravascular ultrasound study. Circulation. 1995;92(12):3408-3414.
- Adamian M, Colombo A, Briguori C, et al. Cutting balloon angioplasty for the treatment of in-stent restenosis: a matched comparison with rotational atherectomy, additional stent implantation and balloon angioplasty. J Am Coll Cardiol. 2001;38(3):672-679.
- Albiero R, Nishida T, Karvouni E, et al. Cutting balloon angioplasty for the treatment of in-stent restenosis. Catheter Cardiovasc Interv. 2000;50(4):452-459.
- Hoffmann R, Mintz GS, Satler LF, et al. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation. 1996;94(6):1247-1254.
- Inoue T, Sakai Y, Hoshi K, et al. Lower expression of neutrophil adhesion molecule indicates less vessel wall injury and might explain lower restenosis rate after cutting balloon angioplasty. Circulation. 1998;97(25):2511-2518.
- Mehran R, Dangas G, Abizaid A, et al. Treatment of focal in-stent restenosis with balloon angioplasty alone versus stenting: short- and long-term results. Am Heart J. 2001;141(4):610-614.
- Mehran R, G. D, Mintz GS, Waksman R, et al. Treatment of in-stent restenosis with excimer laser coronary angioplasty versus rotational atherectomy: comparative mechanisms and results. Circulation. 2000;101(21):2484-2489.
- Kurbaan AS, Foale RA, Sigwart U. Cutting balloon angioplasty for in-stent restenosis. Catheter Cardiovasc Interv. 2000;50(4):480-483.
- Sharma SK, Duvvuri S, Dangas G, etal. Rotational atherectomy for in-stent restenosis: acute and long-term results of the first 100 cases. J Am Coll Cardiol. 1998;32(5):1358-1365.
- Radke PW, Klues HG, Haager PK, et al. Mechanisms of acute lumen gain and recurrent restenosis after rotational atherectomy of diffuse in-stent restenosis: a quantitative angiographic and intravascular ultrasound study. J Am Coll Cardiol. 1999;34(1):33-39.
- Goldberg SL, Berger P, Cohen DJ, et al. Rotational atherectomy or balloon angioplasty in the treatment of intra-stent restenosis: BARASTER multicenter registry. Catheter Cardiovasc Interv. 2000;51(4):407-413.
- Nagai H, Ishibashi-Ueda H, Fujii K. Histology of highly echolucent regions in optical coherence tomography images from two patients with sirolimus-eluting stent restenosis. Catheter Cardiovasc Interv. 2010;75(6):961-963.
- Teramoto T, Ikeno F, Otake H, et al. Intriguing peri-strut low-intensity area detected by optical coherence tomography after coronary stent deployment. Circ J 2010;74(6):1257-1259.
________________________________________________________________
From the Division of Cardiology, Osaka Rosai Hospital, Osaka, Japan.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted March 20, 2012, provisional acceptance given April 17, 2012, final version accepted April 23, 2012
Address for correspondence: Masami Nishino, MD, PhD, FACC, Division of Cardiology, Osaka Rosai Hospital, 1179-3 Nagasone-cho, Sakai-city, Osaka 591-8025, Japan. Email: mnishino@orh.go.jp












