Diagnostic Performance of Pressure Drop Coefficient in Relation to Fractional Flow Reserve and Coronary Flow Reserve
Abstract: Objectives and Background. Functional assessment of coronary lesion severity during cardiac catheterization is conducted using diagnostic parameters like fractional flow reserve (FFR; pressure derived) and coronary flow reserve (CFR; flow derived). However, the complex hemodynamics of stenosis might not be sufficiently explained by either pressure or flow alone, particularly in the case of intermediate stenosis. CDP (ratio of pressure drop across a stenosis to distal dynamic pressure), a non-dimensional index derived from fundamental fluid dynamic principles based on a combination of intracoronary pressure and flow, may improve the functional assessment of coronary lesion severity. Methods. We performed a meta-analysis of seven studies, retrieved from MEDLINE and PubMed, comparing the results of FFR and CFR of the same lesions. Two studies reported functional measurements (pressure and flow) obtained in individual patients. Five studies reported two-dimensional plots of FFR vs CFR. The FFR and CFR data were digitized and corresponding functional measurements were extracted using the reported mean values of hemodynamic data from each of the five studies. The receiver operating characteristic (ROC) curve was used to identify the optimal cut-off point of CDP, which corresponds to the clinically used cut-off values (FFR = 0.80, FFR = 0.75, and CFR = 2.0). Results. CDP correlated significantly with FFR (r = 0.78; P<.001) and had significant diagnostic efficiency (area under the ROC curve = 89%), specificity (83% and 85%), and sensitivity (81% and 76%) at FFR <0.8 and FFR <0.75, respectively. The corresponding cut-off value for CDP to detect FFR <0.80 and FFR <0.75 was at CDP >27.1 and CDP >27.9, respectively. Conclusions. CDP, a functional parameter based on both intracoronary pressure and flow measurements, has close agreement (area under the ROC curve = 89%) with FFR, the most frequently used method for evaluation of coronary stenosis severity.
J INVASIVE CARDIOL 2014;26(5):188-195
Key words: coronary disease, FFR, CFR, catheterization, meta-analysis
__________________________
Coronary angiography is the current standard for detecting epicardial coronary artery disease (CAD). However, coronary angiography frequently fails to accurately identify the functional significance of intermediate coronary stenoses. Invasive functional studies from the past 15 years have demonstrated favorable outcomes for decision making in patients with intermediate stenoses, including single, multivessel, left main, bifurcation, and ostial lesions.1,2 Thus, integrating angiography and invasive coronary physiology provides unique functional information (pressure and flow) that complements anatomic (angiographic) evaluation, and facilitates optimal decision making for treatment of CAD in the catheterization laboratory.
Assessment of functional coronary lesion severity, using sensor-equipped guidewires, has emerged as a standard diagnostic modality to provide objective evidence of myocardial ischemia during cardiac catheterization.3,4 Coronary diagnostic indices, fractional flow reserve (FFR; pressure derived), and coronary flow reserve (CFR; flow derived) showed a high agreement with non-invasive stress testing.5,6
FFR is an established invasive clinical parameter used for assessing physiological significance of epicardial disease. FFR, defined as the ratio of pressures distal and proximal to a stenosis measured at maximal hyperemia, has a lower bound of ‘0’ representing complete vessel obstruction and an upper bound of ‘1’ representing no obstruction and normal flow. Based on extensive clinical trials, a cut-off value of 0.75-0.80 for FFR was shown to indicate hemodynamic significance of coronary stenosis.2,7,8 Some limitations of FFR include the assumption of zero central venous pressure, as well as its dependence on achieving maximal hyperemia. Failure to achieve peak hyperemia may result in not achieving minimal constant microvascular resistance, leading to underestimation of pressure drop and overestimation of FFR across a stenosis.9
CFR, defined as the ratio of flow at hyperemia to flow at rest, was found to have excellent agreement with non-invasive stress testing at a cut-off value of 2.0.6 An abnormal CFR (<2.0) corresponded to reversible myocardial perfusion defects with high sensitivity and specificity.6 It should be noted that CFR can give the combined effect of epicardial stenosis and microvascular dysfunction, but cannot delineate between the two.
Because these indices, FFR and CFR, are based on either intracoronary pressure or flow, they do not differentiate between hemodynamic status of the epicardial stenosis and microvasculature.10,11 To overcome these issues, parameters were proposed based on simultaneous measurements of pressure drop and velocity. However, these parameters were defined for detection of either epicardial stenosis, namely, hyperemic stenosis resistance index (HSR; ratio of pressure drop across the stenosis to the distal velocity during hyperemia);12 or for the detection of microvascular dysfunction, namely, hyperemic microvascular resistance index (HMR; ratio of mean distal pressure and velocity during hyperemia).13 While FFR and CFR are ratios and do not have any units, HSR and HMR are dimensional quantities with units of mm Hg/cm/s.
Recently, for simultaneous detection of epicardial stenosis and microvascular dysfunction using a single diagnostic parameter, we introduced the functional index, the pressure drop coefficient CDP, the ratio of transstenotic pressure drop (Δp) to distal dynamic pressure (½ × blood density × APV2), where APV (average peak flow velocity) is measured under maximal hyperemia.14 This parameter is a non-dimensional ratio, derived from fundamental fluid dynamic principles that incorporate a simultaneous assessment of pressure drop (Δp) and flow (APV) in its formulation. The functional measurements (Δp and APV) necessary for the evaluation of CDP can be readily obtained during routine cardiac catheterization procedures using a dual-sensor tipped guidewire.
The CDP was validated in in vitro14,15 and in vivo animal studies14-20 to delineate between epicardial stenosis and microvascular dysfunction. Furthermore, in a recent clinical trial,21 CDP has been evaluated in a target patient population to distinguish between stenosis severities.
However, a quantitative evaluation of CDP to correctly predict the epicardial disease and microvascular dysfunction using the preexisting cut-off values of the currently used diagnostic parameters, FFR and CFR, is needed. Thus, we conducted a meta-analysis of the available data from studies reporting both intracoronary pressure (FFR) and flow (CFR) information to determine the limiting value of CDP that can distinguish between patients with epicardial and microvascular dysfunction. We hypothesize that CDP, an index based on combined pressure and flow measurements, has adequate diagnostic accuracy for functional assessment of coronary lesion severity.
Methods
Study eligibility. All English-language studies that have performed both the invasively measured FFR and CFR evaluation for the same coronary lesions were eligible for this analysis. Studies were considered regardless of the clinical setting (asymptomatic, stable angina, unstable angina, after early myocardial infarction [MI], other) and regardless of whether they addressed native coronary arteries or arteries that had undergone PCI (not bypass grafts). Studies with evaluations of the extent of coronary stenosis, only by visual inspection, were deemed ineligible.
Search strategy. We performed a literature search using PubMed, Medline, Embase, Cochrane Central Register of Controlled Trials, Google Scholar, and Internet-based sources of information on clinical trials (www.clinicaltrials.gov, www.tctmd.com, and www.cardiosource.com) for studies written in English from 1990 to 2013 (last updated March 2013). We used the following medical subject headings and search terms: fractional flow reserve; coronary flow reserve; coronary pressure-flow relation; coronary flow; and coronary pressure; in combination with the exploded term “coronary artery disease.” Titles and abstracts were examined, and potentially eligible studies were scrutinized in full text. We reviewed the reference lists of identified articles for additional leads, and selected papers published before 1990 were also considered. Bibliographies of relevant studies and the ‘‘related articles’’ link in PubMed was also used to identify additional studies. We reviewed all eligible reports for potential overlap in the study populations to retain only the largest of the overlapping studies.
Data extraction. For each eligible study, we documented the first author, journal, year of publication, and number of patients evaluated with FFR and CFR. Data pertaining to intracoronary pressure and velocity were extracted from text, tables, and figures. Some studies reported individual hemodynamic data of intracoronary pressure and flow information and the data were directly documented from these studies. Alternatively, some studies reported the two-dimensional plots of FFR vs CFR data. In such scenarios, the corresponding FFR and CFR data were digitized from the published graphs. The corresponding intracoronary pressure (distal to stenosis) and flow (at hyperemia) information was evaluated using the above-mentioned digitized values and the reported mean values of hemodynamic information (mean pressure proximal to stenosis and mean flow at rest) in each study. The hemodynamic information, pressure and flow, thus evaluated, were used to calculate CDP (pressure drop coefficient).
Calculation of CDP. CDP has been previously described in detail in the literature.14,16-18,20 Briefly, CDP is defined as the ratio of transstenotic pressure drop (Δp = pa – pd) to distal dynamic pressure. The product of blood density (ρ), the square of average peak flow velocity (APV) and a constant value of 0.5, ie, 0.5 × ρ × APV2, is calculated to obtain distal dynamic pressure, measured at hyperemia. Blood density, ρ, does not change significantly at hyperemia, and thus can be assumed to have a constant value (1.05 g/cm3).16,22 Thus, where pa and pd are mean pressures measured proximal and distal to the stenosis at hyperemia, respectively.
Analysis. We examined the diagnostic concordance of CDP vs FFR; CDP vs CFR; and CDP vs FFR and CFR, using receiver operating characteristic (ROC) curve analysis. FFR and CFR will be considered “positive” for values of <0.80 and <2.0, respectively, in the main analysis. Analysis was also performed using a threshold of FFR <0.75.
Statistical analysis. Continuous variables are summarized as mean ± standard error. Analysis of variance was used to analyze the data and assess any significant linear correlations, summarized using both the Pearson (r) and Spearman (ρ) correlation coefficients among CDP, FFR, and CFR. Multiple linear regression analysis was also conducted to compare simultaneous correlations among hemodynamic parameters.
Sensitivity and specificity estimates for each analysis are independently combined across all the studies using random effects that allow for the possibility that sensitivity and specificity estimates may differ across studies.
The standardized mean difference (SMD) was calculated for effect size based on sample size23 and 95% confidence intervals (CIs) for each study, and for the pooled studies using variance analysis.24 Weighted mean difference (WMD) and 95% confidence intervals (CIs) were calculated for continuous data. To assess heterogeneity across the different studies, we used the Cochran Q-test. Heterogeneity was also assessed by means of I2 statistic, as proposed by Higgins et al,25 thus determining the variance across groups as a result of heterogeneity instead of chance. Based on I2 statistic, values of 25%, 50%, and 75% were considered as yielding low, moderate, and high heterogeneity, respectively.25,26
ROC curve analysis was used to compare the diagnostic performance of CDP with FFR and CFR.27 ROC curves were generated, and the area under the curve (AUC) was calculated (MedCalc, version 10.2.0.0). The AUC summarizes the accuracy of a diagnostic test. An area of 1 represents a perfect test, while an area of 0.5 represents a bad test. Accuracy of the CDP to correctly predict the outcome of FFR and CFR was calculated for predefined and clinically used cut-off values (CFR = 2.00; FFR = 0.75; and FFR = 0.80). Results were considered statistically significant when P-value was <.05.
Results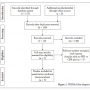
The initial search resulted in 157 titles. Seven studies were included after the review of abstract and examined full text for eligibility (studies reporting both invasively measured FFR and CFR) (Figure 1). Among these, two studies21,28 reported functional measurements (pressure and flow) obtained in individual patients, whereas five studies29-33 reported two-dimensional plots of FFR vs CFR. The hemodynamic information, ie, pressure and flow, was evaluated and then used to calculate CDP.
Combined CFR-FFR measurements. Seven studies matched the selection and search criteria, reporting combined CFR-FFR measurements. The corresponding values of CDP were evaluated from individual pressure drop and flow information. Table 1 summarizes the studies reporting invasive CFR and FFR, along with the number of patients in each study. Figure 2 displays all of the available combined invasive CFR and FFR values (n = 329) evaluated in this study. A significant, but modest, correlation exists between invasive CFR and FFR as a whole (r = 0.41 [95% CI, 0.32-0.50; P<.001]; ρ = 0.37 [95% CI, 0.27-0.46; P<.001]).
reporting combined CFR-FFR measurements. The corresponding values of CDP were evaluated from individual pressure drop and flow information. Table 1 summarizes the studies reporting invasive CFR and FFR, along with the number of patients in each study. Figure 2 displays all of the available combined invasive CFR and FFR values (n = 329) evaluated in this study. A significant, but modest, correlation exists between invasive CFR and FFR as a whole (r = 0.41 [95% CI, 0.32-0.50; P<.001]; ρ = 0.37 [95% CI, 0.27-0.46; P<.001]).
CDP correlations. Figure 3 shows the linear correlation of CDP with FFR and CFR. As shown in Figure 3A, CDP, when correlated with CFR, had a moderate but significant correlation (r = -0.56 [95% CI, -0.63 to -0.48; P<.001]; ρ = -0.65 [95% CI, -0.71 to -0.59; P<.001]). When correlated with FFR, CDP showed a linear and significant correlation (r = -0.78 [95% CI, -0.82 to -0.74; P<.001]; ρ = -0.81 [95% CI: -0.84 to -0.77; P <.001]), as shown in Figure 3B.
P<.001]; ρ = -0.81 [95% CI: -0.84 to -0.77; P <.001]), as shown in Figure 3B.
The functional index, CDP, when correlated simultaneously with FFR and CFR, was found to have a significant and improved correlation (r = 0.82; P<.001), and is shown in Figure 4. This is consistent with the definition of CDP, which is a functional parameter that includes both pressure (FFR) and flow (CFR) information.
Diagnostic characteristics of CDP based on FFR. The ROC curve was used to identify the optimal cut-off point of CDP corresponding to FFR <0.80 and FFR <0.75, as shown in Figure 5. Table 2 summarizes the ROC analysis for CDP to predict FFR <0.75 and FFR <0.80. There were 142 and 181 data points satisfying the criterion for FFR <0.75 and FFR <0.80, respectively.
Table 2 summarizes the ROC analysis for CDP to predict FFR <0.75 and FFR <0.80. There were 142 and 181 data points satisfying the criterion for FFR <0.75 and FFR <0.80, respectively.
The area under the ROC curve for CDP (Figure 5A), to predict FFR <0.75, equals 0.89 (95% CI, 0.85-0.92; P<.001), with a sensitivity of 85.2% and specificity of 76.5%. The corresponding CDP value to predict FFR <0.75 was 27.9. Similarly, the area under the ROC curve for CDP (Figure 5B), to predict FFR <0.80, equals 0.89 (95% CI, 0.85-0.92; P<.001), with a sensitivity of 80.1% and specificity of 83.1%. The corresponding CDP value to predict FFR <0.80 was 27.1. Notably, the ROC curve of CDP yielded a similar AUC for FFR <0.75 and FFR <0.80.
Subgroup analysis of CDP based on CFR. Table 3A shows the subgroup analysis of CDP to detect CFR <2.0 in the FFR <0.75 and FFR >0.75 groups, respectively. In the subgroup of FFR <0.75, the cut-off value to predict CFR <2.0 was CDP >57.9, with a sensitivity of 84.1% and specificity of 93.2%. Similarly, Table 3B shows the subgroup analysis of CDP to detect CFR <2.0 in the FFR <0.80 and FFR >0.80 groups, respectively. In the subgroup of FFR <0.80, the cut-off value to predict CFR <2.0 was CDP >57.9, with a sensitivity of 76.2% and specificity of 93.8%. The corresponding areas under the curve of the ROC curve for the subgroup analysis in FFR <0.75 and FFR <0.80 were significant at 0.93 and 0.90, respectively. In the subgroups of FFR >0.75 and FFR >0.80, the cut-off value to predict CFR <2.0 was a CDP >19.3, with a sensitivity of 63.4% (57.4%) and specificity of 74.1% (85.1%). The corresponding areas under the curve for the ROC curve for the subgroup analysis in FFR <0.75 and FFR <0.80 were significant at 0.75 and 0.76, respectively. Notably, this subgroup analysis (based on FFR) yielded similar cut-off points for CDP to detect CFR, as shown in Table 3. Consolidating the above results, Figure 6 shows the schematic representation of CDP to predict combined assessment of FFR and CFR.
under the curve for the ROC curve for the subgroup analysis in FFR <0.75 and FFR <0.80 were significant at 0.75 and 0.76, respectively. Notably, this subgroup analysis (based on FFR) yielded similar cut-off points for CDP to detect CFR, as shown in Table 3. Consolidating the above results, Figure 6 shows the schematic representation of CDP to predict combined assessment of FFR and CFR.
Discussion
Invasive assessment of pressure drop and coronary flow can provide clinically useful information in patients with known or suspected ischemic heart disease. Recent advances in guidewire technology have introduced dual-sensor pressure and flow wires, which will further simplify the simultaneous assessment of pressure drop and flow. This study has established cut-off values for CDP, in order to predict the outcome of FFR and CFR. This study also showed that the fundamental fluid dynamic diagnostic parameter CDP, which incorporates both pressure drop and flow information in its formulation, correlates well with FFR, which is the most frequently used method for evaluation of coronary stenosis severity.
assessment of pressure drop and flow. This study has established cut-off values for CDP, in order to predict the outcome of FFR and CFR. This study also showed that the fundamental fluid dynamic diagnostic parameter CDP, which incorporates both pressure drop and flow information in its formulation, correlates well with FFR, which is the most frequently used method for evaluation of coronary stenosis severity.
CDP cut-off points predicting the outcome of FFR and CFR. CDP is a fundamental fluid dynamic parameter based on pressure and flow information that can simultaneously distinguish between epicardial stenosis (FFR) and microvascular dysfunction (CFR). The CDP cut-off points to predict FFR <0.75 and FFR <0.80 are shown in Table 2. Similarly, the CDP cut-off points to predict CFR <2.0 in the positive and negative FFR subgroups are shown in Table 3. Consolidating the above results, Figures 6 and 7 show the schematic representation of four different disease combinations based on FFR (epicardial disease) and CFR (microvascular disease).
Table 3. Consolidating the above results, Figures 6 and 7 show the schematic representation of four different disease combinations based on FFR (epicardial disease) and CFR (microvascular disease).
Considering the FFR cut-off of 0.75 as shown in Figure 6, the range of CDP to predict both physiologically insignificant CFR and FFR (FFR >0.75 and CFR >2.0) is between 0 and 19.3, whereas the range of CDP to predict physiologically insignificant FFR and significant CFR (FFR >0.75 and CFR <2.0) is between 19.3 and 27.9. Similarly, the range of CDP to predict physiologically significant FFR and insignificant CFR (FFR <0.75 and CFR >2.0) is 27.9 and 57.9. In this group, the expected physiological interpretation would be the presence of a significant epicardial stenosis (FFR <0.75) and a functionally preserved microcirculation (CFR >2.0). The explanation of the values in this group has to be further investigated, since theoretically CFR should also be affected by the significant epicardial stenosis and its value may not be greater than 2. However, a possible explanation is that some lesions can be detected by FFR, but missed by CFR, because unlike FFR, CFR has a large interindividual variability, influenced by heart rate, contractility, age, gender, and physical training among other factors.34,35 This hypothesis is supported by data from Wilson et al36 and Meuwissen et al,30where highly stenotic lesions were associated with normal CFR values. The values of CDP greater than 57.9 refer to the disease group of physiologically significant FFR and CFR (FFR <0.75 and CFR <2.0). This group can be explained by the presence of significant epicardial stenosis, but the concurrent presence of microvascular disease cannot be excluded. It may be noted that in current clinical practice, for the physiologically significant FFR and CFR disease group, we conclude the presence of microvascular disease in a sequential process of fixing the epicardial lesion and then evaluating whether the CFR is still less than 2. We believe that CDP will be able to diagnose this
significant epicardial stenosis and its value may not be greater than 2. However, a possible explanation is that some lesions can be detected by FFR, but missed by CFR, because unlike FFR, CFR has a large interindividual variability, influenced by heart rate, contractility, age, gender, and physical training among other factors.34,35 This hypothesis is supported by data from Wilson et al36 and Meuwissen et al,30where highly stenotic lesions were associated with normal CFR values. The values of CDP greater than 57.9 refer to the disease group of physiologically significant FFR and CFR (FFR <0.75 and CFR <2.0). This group can be explained by the presence of significant epicardial stenosis, but the concurrent presence of microvascular disease cannot be excluded. It may be noted that in current clinical practice, for the physiologically significant FFR and CFR disease group, we conclude the presence of microvascular disease in a sequential process of fixing the epicardial lesion and then evaluating whether the CFR is still less than 2. We believe that CDP will be able to diagnose this concomitant disease without adopting the sequential process.
concomitant disease without adopting the sequential process.
For an FFR cut-off of 0.80, as shown in Figure 7, the range of CDP to predict both physiologically insignificant CFR and FFR (FFR >0.80 and CFR >2.0) is between 0 and 19.3, whereas the range of CDP to predict physiologically insignificant FFR and significant CFR (FFR >0.80 and CFR <2.0) is between 19.3 and 27.1. Similarly, the range of CDP to predict physiologically significant FFR and insignificant CFR (FFR <0.80 and CFR >2.0) is 27.1 and 57.9, whereas values of CDP >57.9 refer to the disease group of physiologically significant FFR and CFR (FFR <0.80 and CFR <2.0).
Thus, for the present patient population, cut-offs for CDP are obtained from pressure and flow measurements using meta-analysis, to delineate the epicardial disease and microvascular dysfunction.
dysfunction.
Heterogeneity of pooled analysis. Pooled population (Table 1) from different studies might have heterogeneity. The results (for the endpoint FFR <0.80) of the different studies, and the overall standardized mean differences with 95% CIs are shown in the forest plot (Figure 8). The Q-value for the test of heterogeneity is 5.39 (degree of freedom = 6; P=.49). The heterogeneity was also assessed by means of I2 statistic as proposed by Higgins et al25 (determining the variance across groups as a result of heterogeneity instead of random error). The index of inconsistency, I2, represents the percentage of the total variation, which is due to variations between studies. There was no significant heterogeneity with respect to the endpoint of FFR <0.80 and FFR <0.75 (I2 = 0%; P=.43).
Combined measurement of pressure and flow. We have also correlated another diagnostic parameter that combines pressure and flow measurements, HSR (hyperemic stenosis resistance: ratio of transstenotic pressure drop to the distal average peak velocity)12 in relation to CDP. It should be noted that CDP when correlated with HSR (Figure 9), a linearly significant correlation (r=0.97; P<.001) was observed. This is expected, since both CDP and HSR are measures of stenosis resistances as they are functions of both pressure and flow measurements.
ratio of transstenotic pressure drop to the distal average peak velocity)12 in relation to CDP. It should be noted that CDP when correlated with HSR (Figure 9), a linearly significant correlation (r=0.97; P<.001) was observed. This is expected, since both CDP and HSR are measures of stenosis resistances as they are functions of both pressure and flow measurements.
Advantages of pressure drop coefficient. It should be noted that physiologically, the extent of reduction in maximal hyperemic flow due to microvascular dysfunction is higher than that due to epicardial stenosis. In such circumstances, the square of maximal hyperemic flow in the denominator of CDP magnifies this reduction, thus providing an increased resolving power for CDP. This allows improved delineation of the status of epicardial vessel and microcirculation simultaneously. Theoretically, the values of CDP range from zero to infinity. Furthermore, it should be noted that in the presence of microvascular dysfunction and submaximal hyperemia, pressure drop and blood flow are affected in the same direction. In such scenarios, as mentioned previously, diagnostic indices based on either pressure or flow alone can be affected significantly. CDP, however, is a diagnostic parameter based on both pressure and flow information, and is thus expected to be unaffected and show a similar trend. Thus, in the future, we plan to evaluate the effect of hyperemia on CDP by comparing the CDP measured under hyperemia and baseline conditions (without needing hyperemia).
Study limitations. Seven studies were included in this meta-analysis. Among these, two studies (group 1)21,28 reported functional measurements (pressure and flow) obtained in individual patients; whereas five studies (group 2)29-33 reported two-dimensional plots of FFR vs CFR, allowing digitization of FFR and CFR data from the published graphs. The corresponding intracoronary pressure (distal to stenosis) and flow (at hyperemia) information was evaluated using these digitized values and the reported mean values of hemodynamic information (mean flow at rest and mean pressure proximal to stenosis) in each study. However, to further compare the interpretation of this methodology, we evaluated the weighted average means of the flow values at rest and hyperemia between group 1 and group 2. There was no significant difference (P>.05) in the mean values of the flow at rest (21.33 ± 6.71 cm/s in group 1 vs 20.03 ± 8.35 cm/s in group 2) and hyperemia (39.77 ± 9.47 cm/s in group 1 vs 42.22 ± 14.62 cm/s in group 2). Nonetheless, we believe that patient level meta-analysis of the trials reporting individual hemodynamic data could add to the current analysis.
Conclusion
This meta-analysis based on the available data from studies reporting both intracoronary pressure and flow information determined the diagnostic cut-off values of CDP that can differentiate between the hemodynamic status of epicardial stenosis and microvascular dysfunction. The combined pressure and flow diagnostic parameter, CDP, had a close agreement (area under ROC curve = 89%) with FFR and a good correlation with FFR and CFR. The present data also highlight the need for combined assessment of pressure and flow information for detecting CAD. Therefore, we believe that CDP by combining both velocity and pressure information might be more accurate than FFR or CFR in predicting functional significance of stenosis.
Acknowledgments. The authors would like to thank the registered nurses and staff of the cardiac catheterization laboratories at Cincinnati VA Medical Center and University of Cincinnati Medical Center for their help.
References
- Fearon WF, Tonino PA, De Bruyne B, Siebert U, Pijls NH. Rationale and design of the fractional flow reserve versus angiography for multivessel evaluation (FAME) study. Am Heart J. 2007;154(4):632-636.
- Pijls NH, van Schaardenburgh P, Manoharan G, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER study. J Am Coll Cardiol. 2007;49(21):2105-2111.
- Smith SC Jr, Feldman TE, Hirshfeld JW Jr, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention — summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (ACC/AHA/SCAI writing committee to update the 2001 guidelines for percutaneous coronary intervention). Circulation. 2006;113(1):156-175.
- Wijns W, Kolh PH. Experience with revascularization procedures does matter: low volume means worse outcome. Eur Heart J. 2010;31(16):1954-1957.
- Kern MJ. Coronary physiology revisited: practical insights from the cardiac catheterization laboratory. Circulation. 2000;101(11):1344-1351.
- Kern MJ, Lerman A, Bech JW, et al. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association committee on diagnostic and interventional cardiac catheterization, council on clinical cardiology. Circulation. 2006;114(12):1321-1341.
- Pijls NH, Van Gelder B, Van der Voort P, et al. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995;92(11):3183-3193.
- Silber S, Albertsson P, Aviles FF, et al. Guidelines for percutaneous coronary interventions. The task force for percutaneous coronary interventions of the European Society of Cardiology. Eur Heart J. 2005;26(8):804-847.
- Pijls NH, Kern MJ, Yock PG, De Bruyne B. Practice and potential pitfalls of coronary pressure measurement. Catheter Cardiovasc Interv. 2000;49(1):1-16.
- Hoffman JI. Problems of coronary flow reserve. Ann Biomed Eng. 2000;28(8):884-896.
- van de Hoef TP, Nolte F, Rolandi MC, et al. Coronary pressure-flow relations as basis for the understanding of coronary physiology. J Mol Cell Cardiol. 2012;52(4):786-793.
- Meuwissen M, Siebes M, Chamuleau SA, et al. Hyperemic stenosis resistance index for evaluation of functional coronary lesion severity. Circulation. 2002;106(4):441-446.
- Siebes M, Verhoeff BJ, Meuwissen M, de Winter RJ, Spaan JA, Piek JJ. Single-wire pressure and flow velocity measurement to quantify coronary stenosis hemodynamics and effects of percutaneous interventions. Circulation. 2004;109(6):756-762.
- Sinha Roy A, Back MR, Khoury SF, et al. Functional and anatomical diagnosis of coronary artery stenoses. J Surg Res. 2008;150(1):24-33.
- Peelukhana SV, Back LH, Banerjee RK. Influence of coronary collateral flow on coronary diagnostic parameters: an in vitro study. J Biomech. 2009;42(16):2753-2759.
- Banerjee RK, Ashtekar KD, Effat MA, et al. Concurrent assessment of epicardial coronary artery stenosis and microvascular dysfunction using diagnostic endpoints derived from fundamental fluid dynamics principles. J Invasive Cardiol. 2009;21(10):511-517.
- Kolli KK, Banerjee RK, Peelukhana SV, et al. Influence of heart rate on fractional flow reserve, pressure drop coefficient, and lesion flow coefficient for epicardial coronary stenosis in a porcine model. Am J Physiol Heart Circ Physiol. 2011;300(1):H382-H387.
- Kolli KK, Banerjee RK, Peelukhana SV, et al. Effect of changes in contractility on pressure drop coefficient and fractional flow reserve in a porcine model. J Invasive Cardiol. 2012;24(1):6-12.
- Peelukhana SV, Banerjee RK, Kolli KK, et al. Effect of heart rate on hemodynamic endpoints under concomitant microvascular disease in a porcine model. Am J Physiol Heart Circ Physiol. 2012;302(8):H1563-H1573.
- Peelukhana SV, Kolli KK, Leesar MA, et al. Effect of myocardial contractility on hemodynamic endpoints under concomitant microvascular disease in a porcine model. Heart Vessels. 2014;29(1):97-109. Epub 2013 Apr 30.
- Kolli KK, Helmy T, Peelukhana SV, et al. Functional diagnosis of coronary stenoses using pressure drop coefficient: a pilot study in humans. Catheter Cardiovasc Interv. 2014;83(3):377-385. Epub 2013 Aug 1.
- Banerjee RK, Sinha Roy A, Back LH, Back MR, Khoury SF, Millard RW. Characterizing momentum change and viscous loss of a hemodynamic endpoint in assessment of coronary lesions. J Biomech. 2007;40(3):652-662.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Routledge; 1988.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560.
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845.
- Di Mario C, Gil R, de Feyter PJ, Schuurbiers JC, Serruys PW. Utilization of translesional hemodynamics: comparison of pressure and flow methods in stenosis assessment in patients with coronary artery disease. Cathet Cardiovasc Diagn. 1996;38(2):189-201.
- Tron C, Donohue TJ, Bach RG, et al. Comparison of pressure-derived fractional flow reserve with poststenotic coronary flow velocity reserve for prediction of stress myocardial perfusion imaging results. Am Heart J. 1995;130(4):723-733.
- Meuwissen M, Chamuleau SA, Siebes M, et al. Role of variability in microvascular resistance on fractional flow reserve and coronary blood flow velocity reserve in intermediate coronary lesions. Circulation. 2001;103(2):184-187.
- Werner GS, Ferrari M, Richartz BM, Gastmann O, Figulla HR. Microvascular dysfunction in chronic total coronary occlusions. Circulation. 2001;104(10):1129-1134.
- Akasaka T, Yamamuro A, Kamiyama N, et al. Assessment of coronary flow reserve by coronary pressure measurement: comparison with flow- or velocity-derived coronary flow reserve. J Am Coll Cardiol. 2003;41(9):1554-1560.
- Meuwissen M, Chamuleau SA, Siebes M, et al. The prognostic value of combined intracoronary pressure and blood flow velocity measurements after deferral of percutaneous coronary intervention. Catheter Cardiovasc Interv. 2008;71(3):291-297.
- de Bruyne B, Bartunek J, Sys SU, Pijls NH, Heyndrickx GR, Wijns W. Simultaneous coronary pressure and flow velocity measurements in humans. Feasibility, reproducibility, and hemodynamic dependence of coronary flow velocity reserve, hyperemic flow versus pressure slope index, and fractional flow reserve. Circulation. 1996;94(8):1842-1849.
- Hildick-Smith DJ, Johnson PJ, Wisbey CR, Winter EM, Shapiro LM. Coronary flow reserve is supranormal in endurance athletes: an adenosine transthoracic echocardiographic study. Heart. 2000;84(4):383-389.
- Wilson RF, Johnson MR, Marcus ML, et al. The effect of coronary angioplasty on coronary flow reserve. Circulation. 1988;77(4):873-885.
_________________________________
From the 1Department of Mechanical and Materials Engineering, 2Division of Cardiovascular Disease, and 3Department of Environmental Health, University of Cincinnati, Cincinnati, Ohio; 4Veteran Affairs Medical Center, Cincinnati, Ohio; 5Jet Propulsion Laboratory, California Institute of Technology, Pasadena, California; and 6Division of Cardiovascular Disease, University of Alabama-Birmingham, Alabama.
Funding: This work is supported by financial support from Department of Veteran Affairs through VA Merit Review Grant (I01CX000342-01).
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted October 28, 2013, provisional acceptance given November 25, 2013, final version accepted December 5, 2013.
Address for correspondence: R.K. Banerjee, PhD, PE, Department of Mechanical and Materials Engineering, 598 Rhodes Hall, PO Box 210072, Cincinnati, OH 45221-0072. Email: Rupak.Banerjee@UC.edu












