Developments in Coronary Chronic Total Occlusion Percutaneous Coronary Interventions: 2014 State-Of-The-Art Update
Abstract: Percutaneous coronary intervention (PCI) of chronic total occlusions (CTOs) is a rapidly developing field. In the present review, we summarize the most important CTO PCI related literature published in 2013.
J INVASIVE CARDIOL 2014;26(6):261-266
Key words: percutaneous coronary intervention, chronic total occlusion, techniques, outcomes, complications
________________________________
Percutaneous coronary intervention (PCI) of chronic total occlusions (CTOs) is a rapidly developing field, as evidenced by the publication of two CTO books during 2013: (1) Chronic Total Occlusion: A Guide to Recanalization (R. Waksman and S. Saito, John Wiley & Sons, Oxford); and (2) Manual of Chronic Total Occlusion Interventions: a Step-By-Step Approach (ES Brilakis, Elsevier). In the present review, we sought to summarize the most important CTO-PCI related literature published in 2013.
Prevalence and indications. Jeroudi et al reviewed 1699 consecutive patients who underwent coronary angiography at a Veterans Affairs hospital and reported that the prevalence of CTO among coronary artery disease (CAD) patients with and without prior coronary artery bypass graft (CABG) surgery was 89% and 31%, respectively,1 higher than reported in a recent large Canadian registry (54% and 18.4%, respectively),2 but similar to other prior studies.3,4
Several studies reported the adverse impact of incomplete revascularization on subsequent clinical outcomes.5,6 In the Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) trial, angiographic complete revascularization was achieved in only 52.8% of the PCI patients. Presence of a total occlusion was the strongest independent predictor of incomplete revascularization after PCI (hazard ratio [HR], 2.70; 95% confidence interval [CI], 1.98-3.67; P<.001).6 However, CTO PCI success was only 49.4% in the SYNTAX trial, likely reflecting the limited experience in CTO PCI of many participating centers and the limited availability of CTO crossing techniques (patients were enrolled between 2005 and 2007, before the development of retrograde 7,8 and limited antegrade dissection/reentry 9,10 techniques).
The lack of randomized-controlled trials comparing CTO PCI with optimal medical therapy led to initiation of two large randomized-controlled clinical trials: the Drug-Eluting Stent Implantation Versus Optimal Medical Treatment in Patients With Chronic Total Occlusion (DECISION-CTO) trial (NCT01078051) and the European Study on the Utilization of Revascularization versus Optimal Medical Therapy for the Treatment of Chronic Total Coronary Occlusions (EURO-CTO) trial (NCT01760083). DECISION CTO is assessing whether, compared to optimal medical therapy, CTO PCI will reduce the composite endpoint of all-cause death, myocardial infarction (MI), stroke, and any revascularization at 3 years after randomization. EURO-CTO is examining the hypothesis that PCI with implantation of the Biosensors biolimus-eluting stent plus optimal medical therapy will be superior to optimal medical therapy alone in improving health status at 12-month follow-up and will be non-inferior with respect to the composite of all-cause death/non-fatal MI at 36-month follow-up in patients with a CTO in an epicardial coronary artery >2.5 mm in diameter and chronic stable angina with evidence of ischemia and viability in the territory subtended by the CTO. Results of these studies are anticipated in 2018.
Pathology of CTOs. Sakakura et al performed an autopsy study of 95 CTO lesions from 82 patients.11 The main findings were that negative remodeling of the CTO body was frequent and its frequency increased with increasing CTO duration.11 Furthermore, microchannels (>200 µm in diameter) were very rare, in contrast to previous pathology studies, which included fewer subjects and often subtotal occlusions. Hence, the current thinking that soft polymer jacketed guidewires, such as the Fielder XT wire by Asahi Intecc, are successful due to passage through microchannels is not corroborated. Another finding that supports the increasing use of the retrograde approach in CTO PCI was the more frequent tapering of the distal cap as compared to the proximal cap (79% vs 50%; P<.001).11
Procedural planning. A simple way to assess the lesion-specific difficulty of CTO PCI is using the Multicenter CTO Registry of Japan (J-CTO) score. The score is determined by assigning 1 point to each of 5 variables (previously failed lesion, blunt proximal cap, CTO calcification, severe vessel tortuosity, and occlusion length ≥20 mm), thereby allowing patient classification into 4 groups: easy
(J-CTO score = 0), intermediate (score = 1), difficult (score = 2), and very difficult (score ≥3).12 Nombela-Franco validated the value of the J-CTO score for predicting guidewire crossing within 30 minutes in an independent single-operator cohort; however, unlike previous studies, the J-CTO score was not associated with final success rate.13
Burzotta et al performed a systematic review and meta-analysis of radial vs femoral approach for CTO PCI showing lower success with the radial approach (OR, 0.82; 95% CI, 0.68-0.99: P = .046) despite lower complexity CTO lesions in the radial group.14 A significant learning curve was also observed.14 Therefore, although biradial CTO PCI is feasible at experienced radial centers,13,15-18 bifemoral access appears to be associated with higher success rates. Use of sheathless systems such as the Eaucath system (Asahi Intecc) may enable use of larger guide catheters through radial access and increase radial CTO PCI success rates.19
Rolf et al compared the outcomes of 30 patients in whom coronary computed tomography angiography (CTA) was performed before CTO PCI to the outcomes of 43 patients who did not undergo CTA. PCI success rate was significantly higher in patients who had undergone prior coronary CTA (unmatched: CTA 90% [27/30] vs no CTA 63% [27/43], P=.01; matched: CTA 88% [22/25] vs no CTA 64% [16/25], P=.03).20 Coronary CTA may allow better determination of calcification, lesion length, stump morphology, definition of post-CABG anatomy, and presence of side branches compared to angiography alone.20
Sachdeva et al published two studies that provide important insights on use of fractional flow reserve (FFR) during CTO PCI. In the first study, FFR was measured in 50 CTO target vessels after crossing the occlusion and performing balloon angioplasty with a 1.5 mm balloon. All 50 patients studied had FFR <0.80, even in the presence of well-developed collateral vessels.21 Hence, presence of a well-developed collateral circulation does not imply lack of inducible ischemia, and should not deter operators from recanalizing the occluded vessel if clinically indicated.21 In the second study, FFR was performed in the donor vessel before and after successful CTO PCI, showing that 6 of 9 patients (67%) with baseline donor-vessel ischemic FFR had some resolution of ischemia after CTO PCI.22 A similar increase in donor vessel FFR from 0.81 to 0.93 after successful CTO PCI was observed in a case report by Matsuo et al,23 but not in another case report by Sasai et al.24 Therefore, in patients with CTOs and diseased donor vessels, it may be best to first recanalize the CTO, if possible, since this may obviate the need for donor-vessel PCI.
Wilson et al reported 4 cases in which CTO PCI failed balloon angioplasty was performed; nonetheless, repeat angiography a few weeks or months later demonstrated recanalization of the occlusion, highlighting the potential value of a “preplasty” or “investment” procedure.25
Antegrade wiring. Martin-Yuste described the “distal side-branch” technique. When the guidewire enters a side branch after the CTO distal cap, a Tornus catheter (Asahi Intecc) is advanced into the side branch to create a channel within the CTO, which is subsequently used to direct the guidewire into the distal true lumen.26 This technique builds upon several other techniques that have been described for this clinical scenario, such as the “hairpin-wire” (also called “reversed guidewire”27) technique, that can help redirect a guidewire from the side branch into the main vessel.28,29
Antegrade dissection and reentry. Wilson et al reported 87% technical success (27 of 31 cases) in CTOs due to in-stent restenosis using the CrossBoss catheter (Boston Scientific); the CrossBoss catheter crossed into the distal true lumen in most cases.30 The blunt tip of the CrossBoss catheter can often prevent wire passage outside stent struts, facilitating CTO crossing.
Although the Stingray balloon (Boston Scientific) is considered to require a guide catheter at least 6 Fr in diameter, Wu and Ikari reported for the first time successful CTO PCI through a 5 Fr Ikari left 3.5 transradial guide catheter.31 Takahashi et al reported 2 cases in which an IVUS catheter was inserted into the cardiac vein parallel to the target artery to facilitate antegrade wire crossing (Figure 1).32
Valenti et al reported reocclusion in 16 of 28 patients in whom CTO recanalization was achieved using the Subintimal Tracking and Reentry (STAR) technique.33 Given these poor long-term outcomes, the STAR technique should only be used as a “last resort,” after failure of other techniques and devices to cross the CTO.10
Godino et al reported a case of residual long coronary dissection left unstented after guided STAR in a right coronary artery that had resolved at 2-month follow-up exam, suggesting that sometimes conservative management may be appropriate in areas of coronary dissection, especially if Thrombolysis in Myocardial Infarction (TIMI)-3 flow is present in spite of the dissection and if the dissection persists into distal branches.34
Mogabgab et al reported the short- and long-term outcomes in patients in whom the CrossBoss catheter and Stingray system (Boston Scientific) were used (n = 60) and compared them to patients treated with other techniques (n = 110).35 During a median follow-up of 1.81 years, the CrossBoss/Stingray group had no difference in target lesion revascularization (40.9% vs 29.6%; P=.13) or major adverse clinical events (40.3% vs 35.2%; P=.42) in spite of its use in higher complexity cases.35
Retrograde approach. Several enhancements of the retrograde approach were published in 2013. Mozid et al reported use of a Guideliner catheter to perform reverse controlled antegrade and retrograde tracking and dissection (reverse CART) in a patient in whom the retrograde microcatheter was not long enough to reach the antegrade guide catheter.36 The Guideliner catheter was advanced through the antegrade guide catheter over the retrograde microcatheter (“capture technique”), allowing wire externalization and successful completion of the case.36 Dai et al performed IVUS-guided reverse CART in 49 patients.37 IVUS identified that initially 61.7% of retrograde wires were located in the intimal space, and 59.5% of antegrade wires were located in subintimal space, leading to excellent technical (95.9%) and procedural success (93.9%).37 Kim et al reported 20 successful retrograde CTO PCI cases in which the rendezvous method was used instead of wire externalization.38 In the rendezvous method, after a retrograde guidewire has successfully crossed into the proximal true lumen, a microcatheter is then advanced into the antegrade guiding catheter over the retrograde guidewire, where it is aligned with an antegrade microcatheter (usually at a point of aortic curvature), followed by insertion of an antegrade guidewire into the retrograde microcatheter and advancement distal to the CTO. Whether the rendezvous methods will gain more popularity, however, remains unclear since wire externalization provides superior support for equipment delivery and can easily be accomplished with contemporary long guidewires, such as the Viper, RG3, and R350. Similar to prior reports,39,40 Uehara et al reported retrograde stent delivery through an epicardial collateral after failure of antegrade stent delivery.41 The retrograde approach was used as a bail-out procedure after antegrade crossing failed during primary PCI for ST-segment elevation acute MI, although this should only be performed as a last resort when all other approaches fail.42
Tsuchikane et al reported 84.8% technical success and 83.8% clinical success rates in 801 patients undergoing retrograde CTO PCI between 2009 and 2010 in a Japanese multicenter registry. The use of channel dilators increased in 2010 compared to 2009 (95.3% vs 36%; P < .001), as did the use of the reverse CART technique (41.9 vs 66.5%).43 Finally, Lo et al reported higher incidence of periprocedural myocardial injury (>3x the upper limit of normal increase in CK-MB levels) in patients treated with the retrograde vs the antegrade approach (13.8% vs 6.7%; P=.04).44
Procedural outcomes. A multicenter United States (US) CTO registry of 1361 consecutive native coronary artery CTO PCIs performed at 3 US institutions from January 2006 to November 2011 showed 85.5% and 84.2% technical and procedural success rates, respectively, with a major complication rate of 1.8%.45 The retrograde approach was used in 34% of all procedures and its use increased over time.45
De Felice et al reported that in patients undergoing rescue PCI, presence of a CTO was associated with higher 1-year mortality (HR, 3.4; 95% CI, 1.6-7.1; P=.001).46 Similar findings were reported by Hoebers in ST-segment elevation acute MI patients with and without cardiogenic shock47 and by Gierlotka et al in patients with non-ST segment elevation acute MI.48 Yang et al achieved successful CTO PCI in 64 of 136 patients who had presented with ST-segment acute MI within the past 7-10 days. During 2-year follow-up, cardiac mortality was lower (8.0% vs 20.4%; P=.04) and major adverse event-free survival was higher (78.2% vs 61.2%; P=.04) in patients with successful revascularization vs those with failed revascularization of a CTO in the non-infarct related artery.49
In a single-center experience, Syrseloudis et al reported an increase in attempted CTO lesion complexity over a 10-year period, which may explain the relatively modest improvement in overall procedural success rates, whereas success for each J-CTO score category significantly increased over time.50
Several CTO PCI meta-analyses were published in 2013. Khan et al reported that compared to failed CTO PCI, successful recanalization of a CTO resulted in improved all-cause mortality (relative risk [RR], 0.54; 95% CI, 0.45-0.65; P<.001), lower rates of major adverse cardiac events (RR, 0.70; 95% CI, 0.60-0.83; P<.001) and reduced need for subsequent bypass surgery (RR, 0.25; 95% CI, 0.21-0.30; P<.001).51 Similarly, Pancholy et al reported that successful CTO PCI using a predominantly stent-based strategy was associated with a significant reduction in short- and long-term mortality compared to unsuccessful CTO PCI.52
Godino et al analyzed 1345 consecutive patients who underwent CTO PCI between 1998 and 2008 and found that non-revascularized patients (either because of CTO PCI failure or because CTO PCI was not attempted) had a significantly higher 4-year cardiac mortality (8.5% vs 2.5%; P<.001) and sudden cardiac death (2.7% vs 0.5%; P=.001) compared to revascularized patients.53 The most significant independent predictors of cardiac death were: chronic renal failure (HR, 6.0; 95% CI, 2.66-13.80), low left ventricular ejection fraction (HR, 5.7; 95% CI, 2.84-11.58), and insulin-dependent diabetes mellitus (HR, 4.6; 95% CI, 1.96-10.97).53
“Balloon uncrossable” CTOs. Balloon uncrossable CTOs are lesions that cannot be crossed with a balloon after successful guidewire crossing.29 These lesions are usually approached with a combination of lesion modification techniques (such as use of microcatheters and multiple balloons) and increased guide support (such as use of anchoring techniques and use of guide catheter extensions). Four 2013 publications provided novel insights in this area. Hu et al described the “wire-cutting” technique, in which 2 guidewires (A and B) are inserted into the distal true lumen, followed by advancing a balloon over guidewire A to the site of the occlusion abutting the proximal cap. The balloon is then inflated, pressing guidewire B between the balloon and the proximal cap, followed by rapid withdrawal of guidewire B, which “cuts” the proximal cap, facilitating balloon crossing.54 The weakness of this technique is the need to pass 2 wires into the distal true lumen. Michael et al described the “subintimal distal anchor” technique, in which a second coronary guidewire is advanced through the subintimal space distal to the occlusion site with a balloon subsequently inflated to “anchor” the guidewire that has crossed into the distal true lumen, thus facilitating balloon crossing on the initial wire.55 Kovacic et al reported that use of the Guideliner guide catheter extension (Vascular Solutions) was successful in 24 of 28 (85.7%) balloon uncrossable CTO cases.56 Finally, Fernandez et al reported successful crossing in 13 of 16 (81%) balloon uncrossable CTOs using excimer laser, without any significant complications; in some cases, procedural success was achieved with rotational atherectomy following laser application.57
Stents in CTO PCI. In the Chronic Coronary Occlusion Treated by Everolimus-Eluting Stent (CIBELES) trial, 207 patients (79.7% of whom had CTO duration >3 months) were randomized to sirolimus-eluting stent (SES) or everolimus-eluting stent (EES).58 Follow-up coronary angiography performed at 9 months showed similar late loss with SES and EES (0.29 ± 0.60 vs 0.13 ± 0.69 mm, respectively; P<.01 for non-inferiority) and similar clinical outcomes at 12 months, with a trend for lower stent thrombosis risk in the EES group (3% vs 0%; P=.08).58
Wöhrle and Werner compared 48 patients undergoing CTO PCI with bare-metal stents followed by inflation of a paclitaxel-coated balloon to 48 matched historical controls treated with a paclitaxel-eluting stent.59 Patients treated with BMS and drug-eluting balloons had numerically higher but statistically similar loss and binary restenosis and similarly low rates of target vessel revascularization and stent thrombosis.59 Hence, the BMS and drug-eluting balloon combination does not appear to be an attractive CTO treatment option.60
Valenti et al reviewed the outcomes of 802 consecutive patients who underwent successful CTO PCI at a single center between 2003 and 2010.33 The angiographic follow-up rate was 82%. Reocclusion rate was 7.5%, whereas binary restenosis (>50%) or reocclusion rate was 20%. EESs were associated with a significantly lower reocclusion rate vs other drug-eluting stents (3.0% vs 10.1%; P=.001). As described above, a successful STAR technique was associated with a 57% reocclusion rate. By multivariable analysis, the subintimal tracking and re-entry technique (OR, 29.5; P<.001) and EESs (OR, 0.22; P=.001) were independently related to the risk of reocclusion.
A systematic review of DES implantation in CTOs suggested that second-generation DESs provide improved deliverability and enhanced efficacy and safety, while bioabsorbable stents are likely to 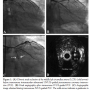 become the next major advancement in the field.61 La Manna et al reported the first case of multiple everolimus-eluting bioresorbable vascular scaffolds (BVS; Absorb, Abbot Vascular) implantation in the right coronary artery, which he named “full polymer jacket.”62 Possible advantages may include restoration of vessel vasomotion, while minimizing the risk for very late stent thrombosis. Yet, questions remain about the impact of BVS scaffolds in longer stented segments, particularly where subintimal tracking techniques have been employed.
become the next major advancement in the field.61 La Manna et al reported the first case of multiple everolimus-eluting bioresorbable vascular scaffolds (BVS; Absorb, Abbot Vascular) implantation in the right coronary artery, which he named “full polymer jacket.”62 Possible advantages may include restoration of vessel vasomotion, while minimizing the risk for very late stent thrombosis. Yet, questions remain about the impact of BVS scaffolds in longer stented segments, particularly where subintimal tracking techniques have been employed.
Optical coherence tomography may be useful in limiting the stent length by determining whether a dissection or coronary lesion is present distal to the CTO segment.29,63
Challenging subgroups. Patients with prior CABG who undergo CTO PCI have lower success rates compared to patients without prior CABG. Among 1363 patients in a multicenter CTO registry, 37% had prior CABG and those patients were older, had more comorbidities, were treated more frequently with the retrograde approach (46.7% vs 27.1%; P<.001), and had lower technical success rates (79.7% vs 88.3%; P=.02), but similar major complication rates (2.1% vs. 1.5%, P=.39) compared to patients without prior CABG.64 Sakakura et al reported that CTOs in CABG patients are characterized by severe calcification and moderate negative remodeling, helping to explain in part the lower CTO PCI success rates in these patients, which may also be influenced by surgically induced distortion of anatomy.11 Although PCI of saphenous vein graft (SVG) CTOs has received a class III recommendation in the American College of Cardiology/American Heart Association PCI guidelines65 because of high restenosis rates,66 Garg et al reported a 79% technical success rate in 28 SVG CTO lesions, with significant improvement in angina among successful cases and similar incidence of adverse events during long-term follow-up.67 Hence, although PCI of a native vessel is preferable to SVG PCI, SVG CTO PCI could be considered in highly selected patients where revascularization to the  ischemic territory is appropriate and the native CTO is technically difficult to recanalize.67 In some cases, a diseased SVG can act as a retrograde conduit for performing native coronary artery CTO PCI.68
ischemic territory is appropriate and the native CTO is technically difficult to recanalize.67 In some cases, a diseased SVG can act as a retrograde conduit for performing native coronary artery CTO PCI.68
Complications of CTO PCI. Patel et al performed a weighted meta-analysis of 65 studies with 18,061 patients and 18,941 target CTO vessels.69 Pooled estimates of outcomes were as follows: angiographic success 77% (95% CI, 74.3%-79.6%); death 0.2% (95% CI, 0.1%-0.3%); emergent CABG surgery 0.1% (95% CI, 0.0%-0.2%); stroke <0.01% (95% CI, 0.0%-0.1%); MI 2.5% (95% CI, 1.9%-3.0%); Q-wave MI 0.2% (95% CI, 0.1%-0.3%); coronary perforation 2.9% (95% CI, 2.2%-3.6%); tamponade 0.3% (95% CI, 0.2%-0.5%); and contrast nephropathy 3.8% (95% CI, 2.4%-5.3%). Compared with successful procedures, unsuccessful procedures had higher rates of death (0.42% vs 1.54%; P<.001), perforation (3.65% vs 10.70%; P<.001), and tamponade (0% vs 1.65%; P <.001). The risk for complications decreased, whereas procedural success increased over time (Figure 2).69 However, a shortcoming of this meta-analysis is the fact that radiation risks and contrast-induced nephropathy were not closely monitored in many of the included reports.
Excessive radiation exposure remains a potential concern for CTO PCI, as for all medical procedures involving radiation exposure. Godino et al estimated that after CTO PCI, the lifetime attributable risk (LAR) of lung and bone marrow cancer was up to 18/100,000 persons exposed and up to 3.5/100,000 persons exposed, respectively.70 While measures to reduce patient and operator exposure should be employed, it is worth considering that such estimates of increased cancer risk are based on linear approximations of historical exposure and as such are impossible to validate.
Lo et al performed systematic cardiac biomarker measurement after CTO PCI and reported that the incidence of periprocedural myocardial injury was higher in patients treated with a primarily retrograde approach compared to an antegrade approach (13.8% vs 6.7%; P=.04).44 Bhattacharyya et al reported formation of a ventricular septal hematoma after retrograde crossing attempts through septal collaterals,71 which was treated conservatively and had almost completely resolved by 10 months post procedure.71 Aggarwal et al described left atrial hematoma formation due to perforation of an epicardial collateral from the circumflex to the right posterior descending artery during retrograde CTO PCI.72 The hematoma extended into the pleural space causing a pleural effusion; impeded filling of the left atrium resulting in “functional” mitral stenosis and compressed the right inferior pulmonary vein, resulting in localized pulmonary edema. A chest tube was placed, which stabilized the patient; this was followed by gradual resolution of the hematoma without surgical intervention, although in similar cases emergent cardiac surgery or CT-guided aspiration may often be required to restore antegrade blood flow. Bagur et al reported use of contrast echocardiography in 4 cases of coronary perforation without overt signs of cardiac tamponade to confirm or exclude active bleeding into the pericardial space when not otherwise visible by conventional imaging measures.73 Patel et al reported a case of inadvertent stent deployment into the subintimal space, without subsequent luminal reentry, leading to ST-segment elevation and no distal outflow.74 A polymer-jacketed guidewire was used to reenter the distal branches of the right coronary artery, restoring antegrade flow and resulting in resolution of ST-segment changes.74
Other. Karmpaliotis et al reported their experience initiating a CTO PCI program. After implementation of quality and performance guidelines, CTO PCI was performed with high success (85.6%) and low complication rates.75 Although procedural and total cost per patient were significantly higher among the CTO cohort, the overall contribution margin was similar with non-CTO PCI ($5173 ± 12,052 vs $5730 ± 8958; P=.58).75 Guo performed virtual histology IVUS in 50 CTO lesions after guidewire crossing and found fibroatheroma in 84% of lesions, suggesting that most CTO lesions develop from acute coronary syndrome and thrombosis and the minority from atherosclerosis progression.76 Sobajima et al reported that repeated sauna therapy improved myocardial perfusion in patients with coronary CTOs.77 Choi performed magnetic resonance imaging in 170 consecutive patients with coronary CTO, showing evidence of prior MI by late gadolinium enhancement in 86%, a much higher proportion that previously recognized, with only 25% of patients showing Q-waves on their electrocardiogram.78
In summary, several developments in CTO PCI appeared during 2013, enhancing our ability to understand and optimally treat these challenging lesions.
References
- Jeroudi OM, Alomar ME, Michael TT, et al. Prevalence and management of coronary chronic total occlusions in a tertiary veterans affairs hospital. Catheter Cardiovasc Interv. 2013 Oct 19 (Epub ahead of print).
- Fefer P, Knudtson ML, Cheema AN, et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59(11):991-997.
- Werner GS, Gitt AK, Zeymer U, et al. Chronic total coronary occlusions in patients with stable angina pectoris: impact on therapy and outcome in present day clinical practice. Clin Res Cardiol. 2009;98(7):435-441.
- Kahn JK. Angiographic suitability for catheter revascularization of total coronary occlusions in patients from a community hospital setting. Am Heart J. 1993;126(3 Pt 1):561-564.
- Garcia S, Sandoval Y, Roukoz H, et al. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta-analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol. 2013;62(16):1421-1431.
- Farooq V, Serruys PW, Garcia-Garcia HM, et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial. J Am Coll Cardiol. 2013;61(3):282-294.
- Thompson CA, Jayne JE, Robb JF, et al. Retrograde techniques and the impact of operator volume on percutaneous intervention for coronary chronic total occlusions an early U.S. experience. JACC Cardiovasc Interv. 2009;2(9):834-842.
- Karmpaliotis D, Michael TT, Brilakis ES, et al. Retrograde coronary chronic total occlusion revascularization: procedural and in-hospital outcomes from a multicenter registry in the United States. JACC Cardiovasc Interv. 2012;5(12):1273-1279.
- Whitlow PL, Burke MN, Lombardi WL, et al. Use of a novel crossing and re-entry system in coronary chronic total occlusions that have failed standard crossing techniques: results of the FAST-CTOs (Facilitated Antegrade Steering Technique in Chronic Total Occlusions) trial. JACC Cardiovasc Interv. 2012;5(4):393-401.
- Michael TT, Papayannis AC, Banerjee S, Brilakis ES. Subintimal dissection/reentry strategies in coronary chronic total occlusion interventions. Circ Cardiovasc Interv. 2012;5(5):729-738.
- Sakakura K, Nakano M, Otsuka F, et al. Comparison of pathology of chronic total occlusion with and without coronary artery bypass graft. Eur Heart J. 2013 Oct 14 (Epub ahead of print).
- Morino Y, Abe M, Morimoto T, et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4(2):213-221.
- Nombela-Franco L, Urena M, Jerez-Valero M, et al. Validation of the h-chronic total occlusion score for chronic total occlusion percutaneous coronary intervention in an independent contemporary cohort. Circ Cardiovasc Interv. 2013;6(6):635-643. Epub 2013 Nov 19.
- Burzotta F, De Vita M, Lefévre T, Tommasino A, Louvard Y, Trani C. Radial approach for percutaneous coronary interventions on chronic total occlusions: technical issues and data review. Catheter Cardiovasc Interv. 2014;83(1):47-57. Epub 2013 Aug 1.
- Taketani Y, Kaneda H, Saito S. Successful coronary intervention for chronic total occlusion using a retrograde approach with biradial arteries. J Invasive Cardiol. 2007;19(9):E281-E284.
- Wu CJ, Fang HY, Cheng CI, et al. The safety and feasibility of bilateral radial approach in chronic total occlusion percutaneous coronary intervention. Int Heart J. 2011;52(3):131-138.
- Rinfret S, Joyal D, Nguyen CM, et al. Retrograde recanalization of chronic total occlusions from the transradial approach; early Canadian experience. Catheter Cardiovasc Interv. 2011;78(3):366-374.
- Egred M. Transradial retrograde recanalization of totally occluded left anterior descending artery using a single 7 fr guiding catheter. J Invasive Cardiol. 2013;25(1):57-60.
- Lin CJ, Fang HY, Chen TH, Wu CJ. Transradial percutaneous coronary intervention for chronic total occlusion using sheathless technique and retrograde approach. Catheter Cardiovasc Interv. 2013;82(3):E206-E210.
- Rolf A, Werner GS, Schuhback A, et al. Preprocedural coronary CT angiography significantly improves success rates of PCI for chronic total occlusion. Int J Cardiovasc Imaging. 2013;29(8):1819-1827.
- Sachdeva R, Agrawal M, Flynn SE, Werner GS, Uretsky BF. The myocardium supplied by a chronic total occlusion is a persistently ischemic zone. Catheter Cardiovasc Interv. 2014;83(1):9-16.
- Sachdeva R, Agrawal M, Flynn SE, Werner GS, Uretsky BF. Reversal of ischemia of donor artery myocardium after recanalization of a chronic total occlusion. Catheter Cardiovasc Interv. 2013;82(4):E453-E458.
- Matsuo H, Kawase Y. Physiological impact of CTO recanalization assessed by coronary pressure measurement: a case report. Catheter Cardiovasc Interv. 2013;82(4):E459-E464.
- Sasai H, Sakakura K, Yuri K, et al. Fractional flow reserve for a mild stenosis on the donor artery to chronic total occlusion. Cardiovasc Interv Ther. 2013;28(2):193-196.
- Wilson WM, Bagnall AJ, Spratt JC. In case of procedure failure: facilitating future success. Intervent Cardiol. 2013;5(5):521-531.
- Martin-Yuste V, Alvarez-Contreras L, Brugaletta S, Sabate M. Distal side-branch technique: a new use for the Tornus® catheter. Cardiovasc Revasc Med. 2014;15(2):97-99. Epub 2013 Sep 12.
- Ide S, Sumitsuji S, Kaneda H, Kassaian SE, Ostovan MA, Nanto S. A case of successful percutaneous coronary intervention for chronic total occlusion using the reversed guidewire technique. Cardiovasc Interv Ther. 2013;28(3):282-286.
- Michael T, Banerjee S, Brilakis ES. Distal open sesame and hairpin wire techniques to facilitate a chronic total occlusion intervention. J Invasive Cardiol. 2012;24(3):E57-E59.
- Brilakis ES, ed. Manual of Coronary Chronic Total Occlusion Interventions. A Step-By-Step Approach. Waltham, MA: Elsevier; 2013.
- Wilson W, Walsh S, Hanratty C, et al. A novel approach to the management of occlusive in-stent restenosis (ISR). EuroIntervention. 2014;9(11):1285-1293.
- Wu EB, Ikari Y. Stingray balloon used in slender percutaneous coronary intervention for chronic total occlusion. J Invasive Cardiol. 2013;25(7):E155-E158.
- Takahashi Y, Okazaki H, Mizuno K. Transvenous IVUS-guided percutaneous coronary intervention for chronic total occlusion: a novel strategy. J Invasive Cardiol. 2013;25(7):E143-E146.
- Valenti R, Vergara R, Migliorini A, et al. Predictors of reocclusion after successful drug-eluting stent-supported percutaneous coronary intervention of chronic total occlusion. J Am Coll Cardiol. 2013;61(5):545-550.
- Godino C, Viani GM, Spagnolo P, Pavon AG, Colombo A, Carlino M. Management of large coronary dissection after STAR. Cardiovasc Revasc Med. 2014;15(1):58-60. Epub 2013 Aug 19.
- Mogabgab O, Patel VG, Michael TT, et al. Long-term outcomes with use of the CrossBoss and Stingray coronary CTO crossing and re-entry devices. J Invasive Cardiol. 2013;25(11):579-585.
- Mozid AM, Davies JR, Spratt JC. The utility of a guideliner catheter in retrograde percutaneous coronary intervention of a chronic total occlusion with reverse cart-the “capture” technique. Catheter Cardiovasc Interv. 2014;83(6):929-932. Epub 2013 Oct 19.
- Dai J, Katoh O, Kyo E, Tsuji T, Watanabe S, Ohya H. Approach for chronic total occlusion with intravascular ultrasound-guided reverse controlled antegrade and retrograde tracking technique: single center experience. J Interv Cardiol. 2013;26(5):434-443.
- Kim MH, Yu LH, Tanaka H, Mitsudo K. Experience with a novel retrograde wiring technique for coronary chronic total occlusion. J Interv Cardiol. 2013;26(3):254-258.
- Bansal D, Uretsky BF. Treatment of chronic total occlusion by retrograde passage of stents through an epicardial collateral vessel. Catheter Cardiovasc Interv. 2008;72(3):365-369.
- Utunomiya M, Katoh O, Nakamura S. Percutaneous coronary intervention for a right coronary artery stent occlusion using retrograde delivery of a sirolimus-eluting stent via a septal perforator. Catheter Cardiovasc Interv. 2009;73(4):475-480.
- Uehara Y, Shimizu M, Yoshimura M. A case with successful retrograde stent delivery via AC branch for tortuous right coronary artery. J Invasive Cardiol. 2013;25(2):E39-E41.
- Patel VG, Zankar A, Brilakis E. Use of the retrograde approach for primary percutaneous coronary intervention of an inferior ST-segment elevation myocardial infarction. J Invasive Cardiol. 2013;25(9):483-484.
- Tsuchikane E, Yamane M, Mutoh M, et al. Japanese multicenter registry evaluating the retrograde approach for chronic coronary total occlusion. Catheter Cardiovasc Interv. 2013;82(5):E654-E661. Epub 2013 Jul 30.
- Lo N, Michael TT, Moin D, et al. Periprocedural myocardial injury in chronic total occlusion percutaneous interventions: a systematic cardiac biomarker evaluation study. JACC Cardiovasc Interv. 2014;7(1):47-54.
- Michael TT, Karmpaliotis D, Brilakis ES, et al. Procedural outcomes of revascularization of chronic total occlusion of native coronary arteries (from a Multicenter United States registry). Am J Cardiol. 2013;112(4):488-492.
- De Felice F, Fiorilli F, Parma A, et al. Effect of multivessel coronary artery disease with or without a concomitant chronic total occlusion on 1-year survival in patients treated with rescue angioplasty. J Invasive Cardiol. 2013;25(2):64-68.
- Hoebers LP, Vis MM, Claessen BE, et al. The impact of multivessel disease with and without a co-existing chronic total occlusion on short- and long-term mortality in ST-elevation myocardial infarction patients with and without cardiogenic shock. Eur J Heart Fail. 2013;15(4):425-432.
- Gierlotka M, Tajstra M, Gasior M, et al. Impact of chronic total occlusion artery on 12-month mortality in patients with non-ST-segment elevation myocardial infarction treated by percutaneous coronary intervention (from the PL-ACS Registry). Int J Cardiol. 2013;168(1):250-254. Epub 2012 Oct 8.
- Yang ZK, Zhang RY, Hu J, Zhang Q, Ding FH, Shen WF. Impact of successful staged revascularization of a chronic total occlusion in the non-infarct-related artery on long-term outcome in patients with acute ST-segment elevation myocardial infarction. Int J Cardiol. 2013;165(1):76-79.
- Syrseloudis D, Secco GG, Barrero EA, et al. Increase in J-CTO lesion complexity score explains the disparity between recanalisation success and evolution of chronic total occlusion strategies: insights from a single-centre 10-year experience. Heart. 2013;99(7):474-479.
- Khan MF, Wendel CS, Thai HM, Movahed MR. Effects of percutaneous revascularization of chronic total occlusions on clinical outcomes: a meta-analysis comparing successful versus failed percutaneous intervention for chronic total occlusion. Catheter Cardiovasc Interv. 2013;82(1):95-107. Epub 2013 Mar 25.
- Pancholy SB, Boruah P, Ahmed I, Kwan T, Patel TM, Saito S. Meta-analysis of effect on mortality of percutaneous recanalization of coronary chronic total occlusions using a stent-based strategy. Am J Cardiol. 2013;111(4):521-525.
- Godino C, Bassanelli G, Economou FI, et al. Predictors of cardiac death in patients with coronary chronic total occlusion not revascularized by PCI. Int J Cardiol. 2013;168(2):1402-1409.
- Hu XQ, Tang L, Zhou SH, Fang ZF, Shen XQ. A novel approach to facilitating balloon crossing chronic total occlusions: the “wire-cutting” technique. J Interv Cardiol. 2012;25(3):297-303.
- Michael TT, Banerjee S, Brilakis ES. Subintimal distal anchor technique for “balloon-uncrossable” chronic total occlusions. J Invasive Cardiol. 2013;25(10):552-554.
- Kovacic JC, Sharma AB, Roy S, et al. GuideLiner mother-and-child guide catheter extension: a simple adjunctive tool in PCI for balloon uncrossable chronic total occlusions. J Interv Cardiol. 2013;26(4):343-350.
- Fernandez JP, Hobson AR, McKenzie D, et al. Beyond the balloon: excimer coronary laser atherectomy used alone or in combination with rotational atherectomy in the treatment of chronic total occlusions, non-crossable and non-expansible coronary lesions. EuroIntervention. 2013;9(2):243-250.
- Moreno R, Garcia E, Teles R, et al. Randomized comparison of sirolimus-eluting and everolimus-eluting coronary stents in the treatment of total coronary occlusions: results from the chronic coronary occlusion treated by everolimus-eluting stent randomized trial. Circ Cardiovasc Interv. 2013;6(1):21-28. Epub 2013 Feb 12.
- Wohrle J, Werner GS. Paclitaxel-coated balloon with bare-metal stenting in patients with chronic total occlusions in native coronary arteries. Catheter Cardiovasc Interv. 2013;81(5):793-799. Epub 2012 Nov 8.
- Garcia S, Brilakis ES. Optimal stenting strategy for coronary chronic total occlusion interventions: what is next? Catheter Cardiovasc Interv. 2013;81(5):800-801.
- Brilakis ES, Kotsia A, Luna M, Garcia S, Abdullah SM, Banerjee S. The role of drug-eluting stents for the treatment of coronary chronic total occlusions. Expert Rev Cardiovasc Ther. 2013;11(10):1349-1358.
- La Manna A, Ohno Y, Attizzani GF, Tamburino C. Successful retrograde recanalization of chronic total coronary occlusion with multiple bioresorbable vascular scaffolds (‘full polymer jacket’): initial experience and rationale. Eur Heart J. 2013;34(37):2925.
- Jaguszewski M, Guagliumi G, Landmesser U. Optical frequency domain imaging for guidance of optimal stenting in the setting of recanalization of chronic total occlusion. J Invasive Cardiol. 2013;25(7):367-368.
- Michael TT, Karmpaliotis D, Brilakis ES, et al. Impact of prior coronary artery bypass graft surgery on chronic total occlusion revascularisation: insights from a multicentre US registry. Heart. 2013;99(20):1515-1518. Epub 2013 Apr 18.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58(24):e44-e122. Epub 2011 Nov 7.
- Al-Lamee R, Ielasi A, Latib A, et al. Clinical and angiographic outcomes after percutaneous recanalization of chronic total saphenous vein graft occlusion using modern techniques. Am J Cardiol. 2010;106(12):1721-1727.
- Garg N, Hakeem A, Gobal F, Uretsky BF. Outcomes of percutaneous coronary intervention of chronic total saphenous vein graft occlusions in the contemporary era. Catheter Cardiovasc Interv. 2014;83(7):1025-1032. Epub 2013 Oct 19.
- Sekiguchi M, Yamazaki M, Kurabayashi M. Retrograde percutaneous coronary intervention via critically degenerated saphenous vein grafts for chronic total occlusion in native coronary arteries. World J Cardiovasc Dis. 2013;03:261-265.
- Patel VG, Brayton KM, Tamayo A, et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc Interv. 2013;6(2):128-136. Epub 2013 Jan 23.
- Godino C, Maccagni D, Pavon AG, et al. Estimating incidence of organ cancer related to PCI radiation exposure in patients treated for acute and chronic total occlusions. J Invasive Cardiol. 2013;25(9):441-445.
- Bhattacharyya S, Khattar R, Lindsay AC, Senior R, Di Mario C. Ventricular septal mass formation after retrograde chronic total occlusion percutaneous coronary intervention. Circulation. 2013;128(19):2167-2168.
- Aggarwal C, Varghese J, Uretsky BF. Left atrial inflow and outflow obstruction as a complication of retrograde approach for chronic total occlusion: report of a case and literature review of left atrial hematoma after percutaneous coronary intervention. Catheter Cardiovasc Interv. 2013;82(5):770-775. Epub 2013 Mar 22.
- Bagur R, Bernier M, Kandzari DE, Karmpaliotis D, Lembo NJ, Rinfret S. A novel application of contrast echocardiography to exclude active coronary perforation bleeding in patients with pericardial effusion. Catheter Cardiovasc Interv. 2013;82(2):221-229.
- Patel VG, Banerjee S, Brilakis ES. Treatment of inadvertent subintimal stenting during intervention of a coronary chronic total occlusion. Intervent Cardiol. 2013;5(2):165-169.
- Karmpaliotis D, Lembo N, Kalynych A, et al. Development of a high-volume, multiple-operator program for percutaneous chronic total coronary occlusion revascularization: procedural, clinical, and cost-utilization outcomes. Catheter Cardiovasc Interv. 2013;82(1):1-8. Epub 2013 Apr 11.
- Guo J, Maehara A, Mintz GS, et al. A virtual histology intravascular ultrasound analysis of coronary chronic total occlusions. Catheter Cardiovasc Interv. 2013;81(3):464-470. Epub 2012 Mar 29.
- Sobajima M, Nozawa T, Ihori H, et al. Repeated sauna therapy improves myocardial perfusion in patients with chronically occluded coronary artery-related ischemia. Int J Cardiol. 2013;167(1):237-243. Epub 2012 Jan 13.
- Choi JH, Chang SA, Choi JO, et al. Frequency of myocardial infarction and its relationship to angiographic collateral flow in territories supplied by chronically occluded coronary arteries. Circulation. 2013;127(6):703-709. Epub 2012 Dec 31.
__________________________
From the 1VA North Texas Healthcare System and University of Texas, Southwestern Medical Center at Dallas, Dallas, Texas; 2Columbia University Medical Center, New York, New York; 3Klinikum Darmstadt, Darmstadt, Germany; 4Forth Valley Royal Hospital, Larbert, United Kingdom; and 5University of Arkansas for Medical Sciences, Central Arkansas Veterans Health System, Little Rock, Arkansas.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors reports are as follows: Dr Brilakis: consulting/speaker honoraria from St Jude Medical, Terumo, Janssen, Sanofi, Asahi, Abbott Vascular, Boston Scientific; research support from Guerbet; spouse is an employee of Medtronic. Dr Karmpaliotis: personal fees from Boston Scientific, Abbott Vascular, Medtronic Vascular, Asahi Intecc, and BridgePoint Medical. Dr Werner: personal fees for Asahi Intecc, Abbott Vascular, Biosensors, Terumo; principal investigator of a randomized trial on the benefit of CTO PCI vs medical therapy conducted by the EURO CTO Club sponsored by Asahi Intecc and Biosensors. Dr Spratt: Dr. Spratt: personal fees from Boston Scientific, Abbott Vascular, and Asahi Intecc. Dr Uretsky: no disclosures reported. Dr Luna: no disclosures reported. Dr Banerjee: research grants from Gilead and the Medicines Company; consultant/speaker honoraria from Covidien and Medtronic; ownership in MDCareGlobal (spouse); intellectual property in HygeiaTel.
Manuscript submitted December 16, 2013, provisional acceptance given March 5, 2014, final version accepted March 7, 2014.
Address for correspondence: Emmanouil S. Brilakis, MD, PhD, Dallas VA Medical Center (111A), 4500 South Lancaster Road, Dallas, TX 75216. Email: esbrilakis@gmail.com












