Delayed Left Main Stem Obstruction Following Successful TAVI with an Edwards SAPIEN XT Valve: Successful Resuscitation and Percutaneous Coronary Intervention Using a Non-invasive Automated Chest Compression Device (AutoPulse)
Abstract: Acute coronary artery obstruction at the time of device implantation is a recognized, albeit rare, complication of TAVI and is most frequently managed by emergency percutaneous intervention. This complication usually manifests with circulatory collapse due to compromising left ventricular ischemia and is most often observed immediately following valve deployment in the catheter laboratory or in theater. Immediate circulatory support is often necessary. We describe the first report of delayed left main stem obstruction 3.5 hours after successful deployment of a 26 mm Edwards SAPIEN XT valve via transfemoral implantation, with sudden development of circulatory collapse on the ward. Circulatory support was rapidly and effectively instituted with an automated non-invasive cardiac massage device, AutoPulse, that delivers continuous chest compressions. Successful emergency percutaneous intervention was then undertaken to the left main stem to displace a calcified nodule during automated external cardiac massage with the AutoPulse.
J INVASIVE CARDIOL 2012;24(5):224-228
Key words: TAVI, Edwards SAPIEN XT valve, left main stem, AutoPulse
______________________________________________
Transcatheter aortic valve implantation (TAVI) is now an accepted alternative to surgical aortic valve replacement in patients considered at high risk from or with contraindications to surgical valve replacement.
TAVI has a significant learning curve and includes the recognition of uncommon procedure-related complications. Coronary artery obstruction at the time of device implantation is an infrequent but potentially fatal complication1 and in large series has been observed in 0.6% undergoing TAVI.2 Occlusion of the left main stem (LMS) has been most frequently described1,3-8 and has been reported after implantation of both the Edwards SAPIEN8 and Medtronic CoreValve6 transcatheter heart valves (THV). In these cases, external cardiac massage4 or invasive mechanical circulatory support with intra-aortic balloon pump (IABP)3 or the Tandem Heart left ventricular assist device5 has been required during emergency percutaneous intervention.
We describe a case of LMS occlusion 3.5 hours after successful implant of a 26 mm Edwards SAPIEN XT THV via transfemoral access. Occlusion was heralded by sudden hypotension shortly followed by cardiac arrest. Non-invasive circulatory support was rapidly and effectively instituted with a novel automated load-distributing chest compression device (AutoPulse; Zoll Circulation) and successful PCI undertaken during automated chest compression. To our knowledge, coronary artery obstruction occurring late after THV implantation with successful resuscitation and PCI during automated cardiac compression with the AutoPulse has not been reported.
Case Report
 A 76-year-old woman was transferred to our institution with symptomatic, severe aortic stenosis for consideration of urgent surgical aortic valve replacement. She suffered chronic restrictive lung disease (FEV1, 0.63; FVC, 0.71; ratio, 89%), mild coronary artery disease, chronic renal failure (eGFR, 45 mL/min), chronic anemia, and frailty, and had a low body mass index (BMI, 19). Her logistic EuroSCORE was 9.12%. Preprocedural trans-thoracic echocardiography demonstrated a tri-leaflet aortic valve with a peak velocity of
A 76-year-old woman was transferred to our institution with symptomatic, severe aortic stenosis for consideration of urgent surgical aortic valve replacement. She suffered chronic restrictive lung disease (FEV1, 0.63; FVC, 0.71; ratio, 89%), mild coronary artery disease, chronic renal failure (eGFR, 45 mL/min), chronic anemia, and frailty, and had a low body mass index (BMI, 19). Her logistic EuroSCORE was 9.12%. Preprocedural trans-thoracic echocardiography demonstrated a tri-leaflet aortic valve with a peak velocity of 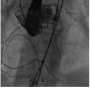 5.8 m/s, mean gradient of 90 mm Hg, valve area of 0.5 cm2, and moderate impairment of left ventricular systolic function. Transesophageal echo (TEE) confirmed the valve to be heavily calcified with bulky leaflets. Aortic valve annulus measured 24 mm on three-dimensional imaging. She was felt to be at high risk from surgical aortic valve replacement and consequently was referred for TAVI with the Edwards SAPIEN valve. Computed tomography aortogram showed good-caliber iliofemoral vessels with no calcification and mild tortuosity, suitable for access with the 18 Fr E-sheath, which expands to 19 Fr on passage of the THV through the sheath.
5.8 m/s, mean gradient of 90 mm Hg, valve area of 0.5 cm2, and moderate impairment of left ventricular systolic function. Transesophageal echo (TEE) confirmed the valve to be heavily calcified with bulky leaflets. Aortic valve annulus measured 24 mm on three-dimensional imaging. She was felt to be at high risk from surgical aortic valve replacement and consequently was referred for TAVI with the Edwards SAPIEN valve. Computed tomography aortogram showed good-caliber iliofemoral vessels with no calcification and mild tortuosity, suitable for access with the 18 Fr E-sheath, which expands to 19 Fr on passage of the THV through the sheath.
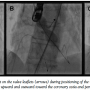
 Under general anesthesia in a cath lab, the right femoral artery was accessed percutaneously and 2 Proglide suture-mediated closure devices (Abbott Vascular) were implanted in a ‘pre-close’ technique. Over an extra-stiff 0.035˝ Amplatz wire, the 18 Fr E-sheath was advanced to the abdominal aorta. The aortic valve was then dilated under burst right ventricular pacing with a 23 mm x 40 mm balloon. During valvuloplasty, it was noted that a calcified nodule on the left aortic leaflet was displaced toward the left coronary ostium (Figures
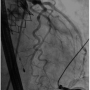
Under general anesthesia in a cath lab, the right femoral artery was accessed percutaneously and 2 Proglide suture-mediated closure devices (Abbott Vascular) were implanted in a ‘pre-close’ technique. Over an extra-stiff 0.035˝ Amplatz wire, the 18 Fr E-sheath was advanced to the abdominal aorta. The aortic valve was then dilated under burst right ventricular pacing with a 23 mm x 40 mm balloon. During valvuloplasty, it was noted that a calcified nodule on the left aortic leaflet was displaced toward the left coronary ostium (Figures  1A-1C). However, the patient remained hemodynamically stable with no electrocardiographic changes and a 26 mm Edwards SAPIEN XT valve (Edwards Lifesciences) was then positioned using fluoroscopic and TEE guidance and deployed, under burst pacing, in a good anatomical position. TEE confirmed a satisfactory position with minimal paravalvular regurgitation and improvement in ventricular function. Aortic root angiography suggested the presence of calcified mass close to, but not obstructing, the left coronary ostium (Figure 2). To examine the LMS more closely, a Judkins left 4 catheter was employed. The catheter did not engage the LMS, but non-selective angiography showed a patent LMS with TIMI 3 flow (Figure 3). As the patient remained hemodynamically stable with no electrocardiographic changes, sheaths were removed and the arteriotomy at the femoral artery was closed successfully with the 2 Proglide sutures. Following reversal of anesthesia, the patient was extubated uneventfully and transported to the nearby coronary care unit (CCU) for close observation.
1A-1C). However, the patient remained hemodynamically stable with no electrocardiographic changes and a 26 mm Edwards SAPIEN XT valve (Edwards Lifesciences) was then positioned using fluoroscopic and TEE guidance and deployed, under burst pacing, in a good anatomical position. TEE confirmed a satisfactory position with minimal paravalvular regurgitation and improvement in ventricular function. Aortic root angiography suggested the presence of calcified mass close to, but not obstructing, the left coronary ostium (Figure 2). To examine the LMS more closely, a Judkins left 4 catheter was employed. The catheter did not engage the LMS, but non-selective angiography showed a patent LMS with TIMI 3 flow (Figure 3). As the patient remained hemodynamically stable with no electrocardiographic changes, sheaths were removed and the arteriotomy at the femoral artery was closed successfully with the 2 Proglide sutures. Following reversal of anesthesia, the patient was extubated uneventfully and transported to the nearby coronary care unit (CCU) for close observation.
 She remained hemodynamically stable for 3.5 hours when she became abruptly hypotensive and breathless. Immediate trans-thoracic echocardiogram excluded pericardial effusion, but showed severe hypokinesia of the antero-lateral walls of the left ventricle. Abdomen was soft with no evidence of blood loss at femoral access sites. She then developed cardio-respiratory arrest and manual cardiopulmonary resuscitation (CPR) was commenced. LMS occlusion was now felt to be the most likely explanation for acute decompensation and emergency
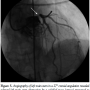
She remained hemodynamically stable for 3.5 hours when she became abruptly hypotensive and breathless. Immediate trans-thoracic echocardiogram excluded pericardial effusion, but showed severe hypokinesia of the antero-lateral walls of the left ventricle. Abdomen was soft with no evidence of blood loss at femoral access sites. She then developed cardio-respiratory arrest and manual cardiopulmonary resuscitation (CPR) was commenced. LMS occlusion was now felt to be the most likely explanation for acute decompensation and emergency 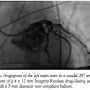 PCI was planned. To facilitate transfer and angiography, CPR using an automated load distributing chest compression device (Autopulse, Zoll Circulation) was commenced. The patient was emergently intubated and transported to the catheter laboratory. The AutoPulse gave excellent hemodynamic support (Figure 4) as compared with no support, assessed by invasive arterial pressure monitoring, and allowed uninterrupted CPR during transfer to the catheter laboratory table. Coronary angiography revealed subtotal occlusion of the LMS by a calcified mass with TIMI 2 flow (Figure 5). During automated chest compressions with AutoPulse, angioplasty guidewires were positioned in the left anterior descending and circumflex arteries and after predilatation with a 2.5 mm x 15 mm compliant balloon the LMS was stented with a 4 mm x 12 mm Integrity Resolute device (Medtronic) (Figure 6). This immediately restored TIMI 3 flow in the left coronary artery and was followed by return of spontaneous circulation, allowing removal of the AutoPulse. The total period of cardiac support with AutoPulse was 38 minutes. Intravascular ultrasound (Boston Scientific) revealed a calcified mass indenting the stent lumen at the mid and proximal section. Postdilatation with a 5 mm non-compliant balloon led to improvement in the angiographic appearance of the stent. Repeat intravascular ultrasound examination demonstrated improvement in minimum lumen area and diameter, but mild residual indentation at the site of the
PCI was planned. To facilitate transfer and angiography, CPR using an automated load distributing chest compression device (Autopulse, Zoll Circulation) was commenced. The patient was emergently intubated and transported to the catheter laboratory. The AutoPulse gave excellent hemodynamic support (Figure 4) as compared with no support, assessed by invasive arterial pressure monitoring, and allowed uninterrupted CPR during transfer to the catheter laboratory table. Coronary angiography revealed subtotal occlusion of the LMS by a calcified mass with TIMI 2 flow (Figure 5). During automated chest compressions with AutoPulse, angioplasty guidewires were positioned in the left anterior descending and circumflex arteries and after predilatation with a 2.5 mm x 15 mm compliant balloon the LMS was stented with a 4 mm x 12 mm Integrity Resolute device (Medtronic) (Figure 6). This immediately restored TIMI 3 flow in the left coronary artery and was followed by return of spontaneous circulation, allowing removal of the AutoPulse. The total period of cardiac support with AutoPulse was 38 minutes. Intravascular ultrasound (Boston Scientific) revealed a calcified mass indenting the stent lumen at the mid and proximal section. Postdilatation with a 5 mm non-compliant balloon led to improvement in the angiographic appearance of the stent. Repeat intravascular ultrasound examination demonstrated improvement in minimum lumen area and diameter, but mild residual indentation at the site of the 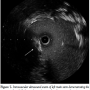 calcified mass (Figure 7). Peak total creatinine kinase was 789 IU/L (ref, <170 IU/L) indicating relatively modest cardiac injury. Echocardiogram showed good left ventricular function, no impairment of prosthesis function or deformation, and importantly no increase in the minor degree of paravalvular regurgitation seen on TEE immediately post deployment. She recovered well from the procedure, with no focal neurology and intact higher mental function and was discharged to home on day 11 post THV feeling symptomatically improved.
calcified mass (Figure 7). Peak total creatinine kinase was 789 IU/L (ref, <170 IU/L) indicating relatively modest cardiac injury. Echocardiogram showed good left ventricular function, no impairment of prosthesis function or deformation, and importantly no increase in the minor degree of paravalvular regurgitation seen on TEE immediately post deployment. She recovered well from the procedure, with no focal neurology and intact higher mental function and was discharged to home on day 11 post THV feeling symptomatically improved. 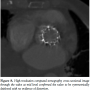 A high-resolution chest computed tomography scan undertaken 21 days post TAVI for investigation of airways disease confirmed the valve to be symmetrically deployed with no evidence of stent deformation (Figure 8). She remains well with good effort capacity (New York Heart Association class 1) and free from further major adverse cardiac events at a follow-up of 89 days.
A high-resolution chest computed tomography scan undertaken 21 days post TAVI for investigation of airways disease confirmed the valve to be symmetrically deployed with no evidence of stent deformation (Figure 8). She remains well with good effort capacity (New York Heart Association class 1) and free from further major adverse cardiac events at a follow-up of 89 days.
Discussion
Acute coronary artery obstruction is a rare but recognized complication of TAVI, with a reported incidence of 0.6%-4.1%2,7 in large- and medium-sized registries. Previous reports of acute coronary obstruction following TAVI have described sudden hemodynamic compromise shortly after device deployment and before return to the ward.3,4,6,8 In all previous reports, emergency PCI was attempted as part of the resuscitation effort. Circulatory collapse is frequently observed with LMS occlusion necessitating immediate institution of circulatory support. Manual CPR,7 Tandem Heart,5 and balloon counterpulsation3 have all been employed. Compared with mechanical circulatory devices, manual CPR provides relatively poor circulatory support, is difficult to perform during concomitant PCI, and puts the resuscitation team at unnecessary risk from harmful ionizing radiation. A further concern with manual CPR during resuscitation, with a functioning Edwards SAPIEN prosthesis in situ, is the serious risk of valve deformation and subsequent malfunction that can arise with chest wall compressions.9 Drawbacks of the Tandem Heart and balloon counterpulsation are that they require time to set up and are invasive, thereby adding additional risk to an emergency situation. In the case described, AutoPulse took less than 30 seconds to implement and supported the patient continuously during transfer and emergency PCI.
 The AutoPulse is a novel, fully automated external cardiac compression band. The device consists of a constricting band and half backboard (Figure 9), which is powered by an exchangeable, rechargeable battery pack that powers the unit during continuous use for up to 45 minutes. The device measures chest size and resistance before it delivers the unique combination of thoracic and cardiac chest compressions. The compression depth and force varies per patient and chest displacement equals a 20% reduction in the anterior-posterior chest depth. The limited and controlled deformation of the chest may minimize the risk of THV deformation previously reported with manual CPR.9 The device is simple to use and can be quickly and effectively used by staff unfamiliar with its operation.10 In the case described, the AutoPulse took less than 30 seconds to place and activate. Compared with manual external massage, the device affords greater hemodynamic support with larger improvements in diastolic, systolic, and mean arterial pressure during cardiac arrest.11 The device is mainly radiolucent and can therefore be employed during PCI. Some alteration in viewing angle may be required because of the electronic components of the AutoPulse, but cranial and caudal projections were not compromised by the device in the case described.
The AutoPulse is a novel, fully automated external cardiac compression band. The device consists of a constricting band and half backboard (Figure 9), which is powered by an exchangeable, rechargeable battery pack that powers the unit during continuous use for up to 45 minutes. The device measures chest size and resistance before it delivers the unique combination of thoracic and cardiac chest compressions. The compression depth and force varies per patient and chest displacement equals a 20% reduction in the anterior-posterior chest depth. The limited and controlled deformation of the chest may minimize the risk of THV deformation previously reported with manual CPR.9 The device is simple to use and can be quickly and effectively used by staff unfamiliar with its operation.10 In the case described, the AutoPulse took less than 30 seconds to place and activate. Compared with manual external massage, the device affords greater hemodynamic support with larger improvements in diastolic, systolic, and mean arterial pressure during cardiac arrest.11 The device is mainly radiolucent and can therefore be employed during PCI. Some alteration in viewing angle may be required because of the electronic components of the AutoPulse, but cranial and caudal projections were not compromised by the device in the case described.
In the reported case, the patient experienced acute and unexpected cardiac arrest on the coronary care unit and had to be urgently transferred back to the lab in order for emergency PCI to be performed. By providing uninterrupted circulatory support during transfer to the cath lab and subsequent PCI, the AutoPulse was likely pivotal in the patient’s survival and intact neurological well being. The effectiveness and safety of the AutoPulse has been described among individuals suffering out of hospital cardiac arrest;12 however, its use during concomitant PCI has not been previously reported in the literature.
Concerning the management of coronary occlusion after TAVI, previous reports have recommended simultaneous aortography during aortic valvuloplasty and routine aortography after valve deployment to examine for the coronary obstruction.7 In the case described, simultaneous aortography during balloon valvuloplasty was not performed. However, aortography and non-selective coronary angiography performed post device deployment did not show flow limitation in the LMS, although on careful inspection some pallor of the LMS was evident. Proposed risk factors for coronary occlusion following TAVI include: (i) narrow aortic root: (ii) shallow sinuses of Valsalva; (iii) low position of coronary ostia (<12 mm from basal leaflet insertion); (iv) pre-existing LMS atheroma; (v) bulky, heavily calcified aortic valve cusps; and (vi) larger valve sizes. However, the individual predictive value of any one or combination of these measurements/observations has not been proven and LMS obstruction remains a largely unpredictable occurrence.
The present case serves to highlight the fundamental importance of close cardiac monitoring of patients post TAVI, especially during the early postprocedural phase, in order that complications are promptly recognized and effectively managed. Furthermore, this case highlights the utility of the AutoPulse in achieving effective and rapid circulatory support post cardiac arrest and is evidence of its compatibility with concomitant emergency PCI in the catheter laboratory. The controlled deformation of the chest wall with the AutoPulse may also reduce the risk of THV deformation which has been described with manual CPR.
References
- Webb JG, Chandavimol M, Thompson CR, et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation. 2006;113(6):842-850.
- Thomas M, Schymik G, Walther T, et al. Thirty-day results of the SAPIEN aortic bioprosthesis European outcome (SOURCE) registry: a European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2010;122(1):62-69.
- Bartorelli AL, Andreini D, Sisillo E, Tamborini G, Fusari M, Biglioli P. Left main coronary artery occlusion after percutaneous aortic valve implantation. Ann Thorac Surg. 2010;89(3):953-955.
- Crimi G, Passerone G, Rubartelli P. Trans-apical aortic valve implantation complicated by left main occlusion. Catheter Cardiovasc Interv. 2011;78(4):656-659.
- Kapadia SR, Svensson L, Tuzcu EM. Successful percutaneous management of left main trunk occlusion during percutaneous aortic valve replacement. Catheter Cardiovasc Interv. 2009;73(7):966-972.
- Saia F, Marrozzini C, Marzocchi A. Displacement of calcium nodules of the native valve as a possible cause of left main occlusion following transcatheter aortic valve implantation. J Invasive Cardiol. 2011;23(5):E106-E109.
- Stabile E, Sorropago G, Cioppa A, et al. Acute left main obstructions following TAVI. EuroIntervention. 2010;6(1):100-105.
- Winther S, Christiansen EH, Thuesen L. Stenting of acute left main coronary artery occlusion using balloon anchoring technique after transcatheter aortic valve implantation. J Interv Cardiol. 2011;24(5):470-473.
- Scherner M, Madershahian N, Strauch JT, Wippermann J, Wahlers T. Transapical valve implantation and resuscitation: risk of valve destruction. Ann Thorac Surg. 2011;92(5):1909-1910.
- Lapostolle F, Agostinucci JM, Bertrand P, et al. Use of an automated device for external chest compressions by first-aid workers unfamiliar with the device: a step toward public access? Acad Emerg Med. 2009;16(12):1374-1377.
- Duchateau FX, Gueye P, Curac S, et al. Effect of the AutoPulse automated band chest compression device on hemodynamics in out-of-hospital cardiac arrest resuscitation. Intensive Care Med. 2010;36(7):1256-1260.
- Krep H, Mamier M, Breil M, Heister U, Fischer M, Hoeft A. Out-of-hospital cardiopulmonary resuscitation with the AutoPulse system: a prospective observational study with a new load-distributing band chest compression device. Resuscitation. 2007;73(1):86-95.
______________________________________________
From the Department of Interventional Cardiology, Queen Elizabeth University Hospital, Birmingham, United Kingdom.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted December 20, 2011, provisional acceptance given January 9, 2012, final version accepted January 20, 2012.
Address for correspondence: Dr. Sagar N. Doshi, Queen Elizabeth University Hospital, Edgbaston, Birmingham B15 2TH. Email: Sagar.Doshi@uhb.nhs.uk












