Coronary Embolus Complicating Peripartum Cardiomyopathy
ABSTRACT: Peripartum cardiomyopathy is a rare disorder in which heart failure occurs during the last month of pregnancy or within 5 months of delivery, in the absence of any other etiology or prior heart disease.1 We present the case of a 42-year-old woman with peripartum cardiomyopathy. She was admitted with an acute myocardial infarction. Multiple mobile ventricular thrombi were seen in the echocardiogram. Coronary angiogram showed consequential coronary embolus occluding the left anterior descending artery. A successful embolectomy was performed followed by coronary stenting. There have been only two reports in the medical literature of coronary embolic events in the setting of peripartum cardiomyopathy;2,3 however, to our knowledge, we believe our case is the first to describe coronary intervention as treatment for the event.
J INVASIVE CARDIOL 2011;23(10):E237–E240
_______________________________________
It is not uncommon to have thromboembolic phenomenon in the course of peripartum cardiomyopathy (PPCM); however, acute myocardial infarction secondary to emboli to the coronary arteries is extremely rare, yet carries devastating consequences. We report this unusual condition in a postpartum young woman as a first presenting symptom, further establishing this disease course as a serious risk factor for acute myocardial infarction.
Case Report
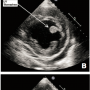 A 42-year-old African American woman presented with a past medical history significant for hypertension. She had an uncomplicated delivery 4 months prior following an uneventful first pregnancy. The patient presented to the emergency department with complaints of nausea, vomiting, and worsening shortness of breath for the past 24 hours. She denied any chest pain symptoms. The patient reported that similar episodes (also with no chest pain) occurred three times over the past few months. There was no family history of cardiomyopathy or premature coronary artery disease, nor had she any congenital heart disease. She was a non-smoker and denied use of illicit drugs. Physical examination revealed S3 and S4 heart sounds, bilateral lung-base crackles, and a body mass index of 36.6. An electrocardiogram demonstrated left bundle branch block with no previous documentation. Laboratory findings included troponin I and creatine kinase levels at 5.55 ng/ml and 60 U/L, respectively, CK-MB fraction <0.5 ng/ml, with a BNP level of 1440 pg/ml. Chest x-ray revealed marked cardiomegaly and pulmonary congestion. An echocardiogram (Figure 1) showed severe diffuse reduction in systolic function and global hypokinesia, an estimated ejection fraction of 15%, and multiple mobile left ventricular mural thrombi. In addition, the patient had an enlarged left ventricle (end-diastolic diameter, 7.4 cm), mildly enlarged left atrium, mild tricuspid regurgitation, and a small pericardial effusion without evidence of tamponade. The patient started becoming hypotensive and developed respiratory failure requiring intubation and ventilator support. She was taken emergently to the cath lab. Coronary angiography (Figure 2) showed an embolus causing an approximately 13 mm area of stenosis and 90% eccentric occlusion of the distal left anterior descending (LAD) artery; the other coronary arteries appeared normal.
A 42-year-old African American woman presented with a past medical history significant for hypertension. She had an uncomplicated delivery 4 months prior following an uneventful first pregnancy. The patient presented to the emergency department with complaints of nausea, vomiting, and worsening shortness of breath for the past 24 hours. She denied any chest pain symptoms. The patient reported that similar episodes (also with no chest pain) occurred three times over the past few months. There was no family history of cardiomyopathy or premature coronary artery disease, nor had she any congenital heart disease. She was a non-smoker and denied use of illicit drugs. Physical examination revealed S3 and S4 heart sounds, bilateral lung-base crackles, and a body mass index of 36.6. An electrocardiogram demonstrated left bundle branch block with no previous documentation. Laboratory findings included troponin I and creatine kinase levels at 5.55 ng/ml and 60 U/L, respectively, CK-MB fraction <0.5 ng/ml, with a BNP level of 1440 pg/ml. Chest x-ray revealed marked cardiomegaly and pulmonary congestion. An echocardiogram (Figure 1) showed severe diffuse reduction in systolic function and global hypokinesia, an estimated ejection fraction of 15%, and multiple mobile left ventricular mural thrombi. In addition, the patient had an enlarged left ventricle (end-diastolic diameter, 7.4 cm), mildly enlarged left atrium, mild tricuspid regurgitation, and a small pericardial effusion without evidence of tamponade. The patient started becoming hypotensive and developed respiratory failure requiring intubation and ventilator support. She was taken emergently to the cath lab. Coronary angiography (Figure 2) showed an embolus causing an approximately 13 mm area of stenosis and 90% eccentric occlusion of the distal left anterior descending (LAD) artery; the other coronary arteries appeared normal.
 Thrombectomy utilizing a Pronto™ catheter (Vascular Solutions, Inc.) of the distal LAD thrombus was performed, followed by balloon angioplasty at the site of the lesion; however, residual stenosis was still evident. A Multi-Link Mini Vision® bare-metal coronary stent (Abbott Vascular) was deployed. Flow improved to TIMI grade-3 following stent placement. A Swan-Ganz right heart catheter was inserted, and later an intra-aortic balloon pump was utilized due to persistent hypotension and cardiogenic shock requiring vasopressor support.
Thrombectomy utilizing a Pronto™ catheter (Vascular Solutions, Inc.) of the distal LAD thrombus was performed, followed by balloon angioplasty at the site of the lesion; however, residual stenosis was still evident. A Multi-Link Mini Vision® bare-metal coronary stent (Abbott Vascular) was deployed. Flow improved to TIMI grade-3 following stent placement. A Swan-Ganz right heart catheter was inserted, and later an intra-aortic balloon pump was utilized due to persistent hypotension and cardiogenic shock requiring vasopressor support.
Anticoagulation with heparin bridging into oral anticogulants was started. The patient was closely monitored in the cardiac care unit until she became hemodynamically stable, and later discharged home on optimal medical management after a 14-day course in the hospital. Successful implantation of a biventricular cardioverter defibrillator ensued thereafter. The patient is undergoing evaluation for left ventricular assist device (LVAD) placement.
Discussion
PPCM is a potentially life-threatening condition, defined as a new onset heart failure between the last month of pregnancy and the first 5 months of the postpartum period;4 absence of identifiable cause for the heart failure or pre-existing heart disease prior to the last month of pregnancy, essentially established by echocardiographic finding of left ventricular dysfunction.1,5 Although considered idiopathic, proposed mechanisms suggest unbalanced oxidative stress and a cardiotoxic Prolactin molecule as key players in the pathophysiology of this disease.6 The incidence of PPCM ranges from 1:1000 to 1:4000 live births, and it affects 1000 to 1300 women in the United States each year.7,8 PPCM is associated with a high maternal morbidity and mortality, reportedly accounting for 4% of maternal deaths in the United States.5,9 African-American race, advanced maternal age (>30 years old), twin pregnancy, use of tocolytics, gestational hypertension, and obesity are well-reported risk factors of PPCM.6,7,10 Thromboembolism, although a known complication, is an unusual initial presentation for PPCM,11-14 and yet is interestingly more frequent than those of dilated cardiomyopathy.15 The occurrence of thromboembolism in PPCM may be due to the nature of hypercoagulable state in pregnant individuals brought upon by the increased concentrations of coagulation factors VII, VIII, X, and plasma fibrinogen, as well as enhanced platelet adhesiveness from late pregnancy to 4–6 weeks postpartum.7,10 In addition, the left ventricular dysfunction in PPCM facilitates thrombus formation by causing relative blood stasis in the ventricles.14 Embolism in PPCM has been reported as a cause of blindness, pulmonary embolism, limb ischemia, stroke, as well as mesenteric and visceral ischemia.6 However, in our case, we describe a very rare event where a coronary embolus is arising from left ventricular mural thrombi, setting off an acute myocardial ischemia and infarction. We know of 2 reported cases of such emboli.2,3 Reperfusion with eptifibatide (glycoprotein IIb/IIIa inhibitor) was associated with improvement in flow acutely in one of the cases, yet with persistent heart failure state on long term and the patient required a heart transplant. Our case further verifies this mechanism as a cause of myocardial infarction in the disease process. We believe our case to be the first to describe thrombectomy and coronary intervention as a treatment for this rare condition in the acute setting. Of note, our patient had no chest pain as a presenting symptom, which further demonstrates the discrepancy between symptoms and disease process manifested in PPCM.
In the presence of severe left ventricular systolic dysfunction, initiation of anticoagulation therapy is warranted.5 LV thrombus is reported to predict worse prognosis15 and pre-emptive coumadin anticoagulation therapy might be considered therapeutic16 and even beneficial for primary prevention.
Generally, management of myocardial infarction secondary to thromboembolism includes early revascularization, medical management of heart failure, and anticoagulation therapy.17 Early recognition can limit the extent of myocardial infarction. Prenatal counseling for further pregnancies is challenging, since recovery of left ventricular function gives no guarantee of safety.6
Exact risk of subsequent heart failure is unknown due to the lack of sufficient studies to establish it; nonetheless, it was reported in up to 20% of patients with normalized LV function 6 months postpartum, and in 50% of those with persisting LV impairment.18
Early diagnosis and treatment of PPCM are essential to achieve a favorable outcome, as delayed onset of symptoms following delivery is associated with a poor prognosis.19 Half of PPCM diagnosed patients do well in the 5-month postpartum period, as their LV function is restored to baseline, and have good overall prognosis.1,7,10
In conclusion, patients with PPCM may rarely be predisposed to thromboembolic phenomenon. Presence of multiple ventricular thrombi carry risk of a rare yet detrimental event of embolizing to a coronary artery, which may result in acute myocardial infarction if not prevented and treated early in the disease course. This may be a further indication for early initiation of anticoagulation and intervention to prevent a potential adverse outcome. In fact, aggressive and anticipatory anticoagulation might be beneficial for PPCM patients and outweighs the hemorrhagic risk of anticoagulation, despite having normal sinus rhythm and normal coagulation studies. The incidence of coronary emboli in PPCM remains unknown. Early diagnosis and recognition along with periodic cardiology follow-up are vital; a high clinical index of suspicion, with evidence of severe left ventricular dysfunction, prompt accurate diagnosis and successful therapy. The utility of echocardiography for peripartum screening may be underestimated and would be of higher value if properly integrated, since the clinical diagnosis of cardiac failure in the setting of pregnancy may very well be masked by non-specific symptoms attributable to the physiologic changes with increased hemodynamic load during pregnancy. Further research is needed to determine efficient methods for early detection of cardiac thromboembolic events in pregnant women at risk of PPCM, as well as to better evaluate benefits of aggressive and pre-emptive anticoagulation.
References
- Demakis JG, Rahimtoola SH, Sutton GC, et al. Natural course of peripartum cardiomyopathy. Circulation. 1971;44(6):1053-1061.
- Dickfield T, Gagliardi JP, Marcos J, Russell SD. Peripartum cardiomyopathy presenting as an acute myocardial infarction. Mayo Clin Proc. 2002;77(5):500-501.
- Box LC, Hanak V, Arciniegas JG. Dual coronary emboli in peripartum cardiomyopathy. Tex Heart Inst J. 2004;31(4):442–444.
- Ibebuogu UN, Thornton JW, Reed GL. An unusual case of peripartum cardiomyopathy manifesting with multiple thrombo-embolic phenomena. Thromb J. 2007;5:18.
- Pearson GD, Veille JC, Rahimtoola S, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283(9):1183-1188.
- Pankaj D. Peripartum cardiomyopathy: a review. J Obstet Gynecol India. 2010;60:25-32.
- Lampert MB, Lang RM. Peripartum cardiomyopathy. Am Heart J. 1995;130(4):860-870.
- Ventura SJ, Peters KD, Martin JA, Maurer JD. Births and deaths: United States, 1996. Mon Vital Stat Rep. 1997;46(1 Suppl 2):1-40.
- Veille JC, Zaccaro D. Peripartum cardiomyopathy: summary of an international survey on peripartum cardiomyopathy. Am J Obstet Gynecol. 1999;181(2):315-319.
- Nishi I, Ishimitsu T, Ishizu T, et al. Peripartum cardiomyopathy and biventricular thrombi. Circ J. 2002;66(9):863–865.
- Demakis JG, Rahimtoola SH. Peripartum cardiomyopathy. Circulation. 1971;44(5):964-968.
- Brown CS, Bertolet BD. Peripartum cardiomyopathy: a comprehensive review. Am J Obstet Gynecol. 1998;178(2):409-414.
- Meadows WR. Idiopathic myocardial failure in the last trimester of pregnancy and the puerperium. Circulation. 1957;15(6):903-914.
- Walsh JJ, Burch GE, Black WC, Ferrans VJ, Hibbs RG. Idiopathic myocardiopathy of the puerperium (postpartal heart disease). Circulation. 1965;32:19-31.
- Amos AM, Jaber WA, Russell SD. Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J. 2006;152(3):509-513.
- Hirsh J, Fuster V, Ansell J, Halperin JL; American Heart Association/American College of Cardiology Foundation. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. J Am Coll Cardiol. 2003;41(9):1633-1652.
- Abboud J, Murad Y, Chen-Scarabelli C, Saravolatz L, Scarabelli TM. Peripartum cardiomyopathy: a comprehensive review. Int J Cardiol. 2007;118(3):295-303. Epub 2007 Jan 17.
- Elkayam U, Tummala PP, Rao K, et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001;344(21):1567-1571.
- Ravikishore AG, Kaul UA, Sethi KK, Khalilullah M. Peripartum cardiomyopathy: prognostic variables at initial evaluation. Int J Cardiol. 1991;32(3):377-380.
_______________________________________
From 1the Department of Internal Medicine, University of Illinois in Chicago/Advocate Christ Medical Center, Oak Lawn, Illinois and 2the Division of Cardiology, Advocate Christ Medical Center, Oak Lawn, Illinois.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted January 26, 2011, provisional acceptance given February 24, 2011, final version accepted March 26, 2011.
Address for correspondence: Luay Rifai, MD, Department of Internal Medicine, University of Illinois in Chicago/Advocate Christ Medical Center, 4440 W 95th St., Oak Lawn, IL 60453-2600. Email: LRifai@UIC.edu












