Coronary Artery Aneurysms in Acute Coronary Syndrome: Case Series, Review, and Proposed Management Strategy
Abstract: Coronary artery aneurysm (CAA) is an uncommon clinical finding, with an incidence varying from 1.5%-4.9% in adults, and is usually considered a variant of coronary artery disease (CAD). CAA identified in the context of acute coronary syndrome (ACS) represents a unique management challenge, particularly if the morphology of the CAA is suspected to have provoked the acute clinical syndrome. CAA is associated with thrombus formation due to abnormal laminar flow, as well as abnormal platelet and endothelial-derived pathophysiologic factors within the CAA. Once formed, mural thrombus may potentiate the deposition of additional thrombus within aneurysmal segments. Percutaneous revascularization of CAA has been associated with complications including distal embolization of thrombus, no-reflow phenomenon, stent malapposition, dissection, and rupture. Presently, there are no formal guidelines to direct the management of CAA in patients presenting with ACS; controversies exist whether conservative, surgical, or catheter-based management should be pursued. In this manuscript, we present an extensive review of the existing literature and associated clinical guidelines, and propose a management algorithm for patients with this complex clinical scenario. Armed with this perspective, therapeutic decisions may be tailored to synthesize patient factors and preferences, individualized clinical assessment, and existing American Heart Association/American College of Cardiology guidelines for management of ACS.
J INVASIVE CARDIOL 2014;26(6):283-290
Key words: aneurysm, coronary disease, catheterization, revascularization, infarction
_____________________________
Coronary artery aneurysm (CAA) is defined as coronary dilatation that exceeds the diameter of normal adjacent segments or the diameter of the largest coronary artery by a factor of 1.5.1 The observed incidence of CAA varies from 1.5%-4.9%, with a slight (40.4%) predilection for the right coronary artery.2 The most common etiology of CAA is coronary artery disease (CAD): however, connective tissue diseases, coronary trauma including iatrogenic injury during percutaneous coronary intervention (PCI), infectious arteritis, Kawasaki syndrome, and congenital CAA also represent etiologic factors. While CAA is often discovered incidentally during coronary angiography, known complications associated with CAA include acute coronary syndrome (ACS), thrombosis, embolization, rupture, and vasospasm.1 Management of CAA during ACS may represent a unique clinical challenge, particularly in the context of profound thrombus burden. Controversy exists whether conservative, surgical, or catheter-based management should be considered for this rare clinical scenario. The current literature exploring this topic consists primarily of case reports, as no clinical trials have been performed to date. In this manuscript, we review the clinical presentation, anatomic considerations, and management strategy in 5 patients presenting with CAA and ACS. In addition, we review available case reports, pertinent clinical guidelines, and based on this review, propose an algorithm for the management of patients with this complex clinical scenario.
Case 1. A 38-year-old Caucasian male with past medical history of dyslipidemia, obesity, and obstructive sleep apnea presented to the emergency department with an ACS. Initial electrocardiogram demonstrated normal sinus rhythm with 1.0 mm ST-segment depressions in leads III and aVF. Medical treatment was started including intravenous (IV) heparin, clopidogrel 600 mg, aspirin 325 mg, and atorvastatin 80 mg. On coronary angiography, the left anterior descending (LAD) coronary artery had a large aneurysmal segment in the mid-portion of the vessel immediately distal to a moderate to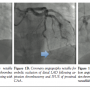 severe stenosis that was difficult to delineate angiographically (Figure 1A). There was TIMI-2 flow in the LAD. The right and left circumflex coronary arteries also contained aneurysmal sections bordered by regions of non-obstructive CAD. Intravascular ultrasound (IVUS; Boston Scientific) was performed, revealing non-critical proximal LAD stenosis and ~8 mm aneurysmal diameter with visible thrombus. Following removal of the coronary guidewire, angiography revealed abrupt total occlusion of the distal LAD, consistent with distal embolization of thrombus (Figure 1B). A BMW coronary guidewire (Abbott Vascular) was advanced across the thrombus; flow was reestablished following manual thrombectomy, administration of intracoronary nicardipine, and serial low-pressure (<6 atm) Apex 2.5 x 15 mm PTCA balloon inflations (Boston Scientific). Final angiography demonstrated a patent LAD with TIMI-3 flow (Figure 1C). The patient was treated with aspirin, clopidogrel, IV unfractionated heparin and IV abciximab (48 hours). Troponin I (reference, <0.05 µg/L) peaked at 10.2 µg/L. The patient remained asymptomatic during the remainder of the hospital course. He was discharged to home on daily aspirin, clopidogrel, metoprolol, and atorvastatin and has done well throughout follow-up.
severe stenosis that was difficult to delineate angiographically (Figure 1A). There was TIMI-2 flow in the LAD. The right and left circumflex coronary arteries also contained aneurysmal sections bordered by regions of non-obstructive CAD. Intravascular ultrasound (IVUS; Boston Scientific) was performed, revealing non-critical proximal LAD stenosis and ~8 mm aneurysmal diameter with visible thrombus. Following removal of the coronary guidewire, angiography revealed abrupt total occlusion of the distal LAD, consistent with distal embolization of thrombus (Figure 1B). A BMW coronary guidewire (Abbott Vascular) was advanced across the thrombus; flow was reestablished following manual thrombectomy, administration of intracoronary nicardipine, and serial low-pressure (<6 atm) Apex 2.5 x 15 mm PTCA balloon inflations (Boston Scientific). Final angiography demonstrated a patent LAD with TIMI-3 flow (Figure 1C). The patient was treated with aspirin, clopidogrel, IV unfractionated heparin and IV abciximab (48 hours). Troponin I (reference, <0.05 µg/L) peaked at 10.2 µg/L. The patient remained asymptomatic during the remainder of the hospital course. He was discharged to home on daily aspirin, clopidogrel, metoprolol, and atorvastatin and has done well throughout follow-up.
Case 2. An 85-year-old man with past medical history of dyslipidemia and atrial fibrillation on chronic warfarin therapy presented with unstable angina. Angiography revealed a dominant left circumflex with aneurysmal dilatation proximal to the bifurcation with the first obtuse marginal branch (OM1). OM1 was large in caliber, with a proximal, thrombus-filled aneurysm (Figure 2) with TIMI-2 flow distally. A CAA was also noted in the proximal LAD with the remainder of the left coronary artery having only mild CAD. The right coronary artery (RCA) was small and non-dominant. Left ventriculography 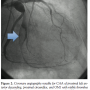 demonstrated an ejection fraction of 20% with severe hypokinesis of the lateral and anterolateral walls. After extensive discussion, the patient declined invasive therapy and elected to proceed with conservative management. IV unfractionated heparin and IV eptifibatide were continued for 48 hours. Atorvastatin, carvedilol, and lisinopril were maximized and warfarin was restarted at discharge with a LMWH bridge, both for paroxysmal atrial fibrillation and thrombosed CAA. He has done well throughout follow-up.
demonstrated an ejection fraction of 20% with severe hypokinesis of the lateral and anterolateral walls. After extensive discussion, the patient declined invasive therapy and elected to proceed with conservative management. IV unfractionated heparin and IV eptifibatide were continued for 48 hours. Atorvastatin, carvedilol, and lisinopril were maximized and warfarin was restarted at discharge with a LMWH bridge, both for paroxysmal atrial fibrillation and thrombosed CAA. He has done well throughout follow-up.
Case 3. A 59-year-old woman with known CAD and chronic obstructive pulmonary disease presented with unstable angina. Ten years previously, she had presented with non-ST elevation myocardial infarction, and underwent successful percutaneous coronary intervention of the distal right coronary artery using a 3.5 x 23 mm Cypher (sirolimus-eluting) stent. On this presentation, coronary angiography disclosed a large aneurysm in the distal RCA, with critical stenosis at the proximal edge of the aneurysm (Figure 3A). Since the focal critical stenosis was felt to be flow-limiting, and culprit in the presentation with unstable angina, and given its proximity to the relatively focal aneurysm, the decision was made to treat the adjacent lesion using a polytetrafluoroethylene (PTFE)-covered stent graft. Following predilatation of the stenosis, a 3.5 x 19 mm Jostent Graftmaster was deployed to cover the aneurysm; in addition, the adjacent proximal  stenosis was covered with an overlapping 3.5 x 18 mm Xience Xpedition (everolimus-eluting) stent (Figure 3B). Final angiography demonstrated exclusion of the aneurysm and no residual stenosis at the adjacent proximal lesion. On the still frame image (Figure 3C), faint opacification with contrast was noted in the aneurysmal space, representing a static pool of contrast trapped at the time of positioning and deployment of the covered stent. IVUS following stent placement demonstrated excellent stent apposition at the inlet and outlet with effective exclusion of the aneurysm (Figure 3D). The patient was managed with life-long aspirin and 1 year of clopidogrel and has done well throughout follow-up.
stenosis was covered with an overlapping 3.5 x 18 mm Xience Xpedition (everolimus-eluting) stent (Figure 3B). Final angiography demonstrated exclusion of the aneurysm and no residual stenosis at the adjacent proximal lesion. On the still frame image (Figure 3C), faint opacification with contrast was noted in the aneurysmal space, representing a static pool of contrast trapped at the time of positioning and deployment of the covered stent. IVUS following stent placement demonstrated excellent stent apposition at the inlet and outlet with effective exclusion of the aneurysm (Figure 3D). The patient was managed with life-long aspirin and 1 year of clopidogrel and has done well throughout follow-up.
Case 4. A 50-year-old man with history of inferior ST-elevation myocardial infarction (STEMI) treated with thrombolytic therapy 17 years prior presented with recurrent inferior STEMI. He was treated with thrombolytic therapy, with resolution of acute ST-elevation and chest pain, and was then transferred to our institution for further risk stratification. Coronary angiography disclosed aneurysmal dilatation with associated thrombus in the RCA (Figure 4A). Filling defects noted in the posterior descending artery and posterolateral left ventricular branches were felt to represent 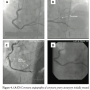 embolization of thrombus from the mid-RCA (Figure 4B). With clinical resolution of the myocardial infarction, TIMI-3 flow in the vessel, and only mild focal stenosis of the mid-RCA, no invasive intervention was undertaken. The patient was managed on aspirin, IV unfractionated heparin, and clopidogrel while in hospital, and was discharged on aspirin and warfarin with the plan for indefinite systemic anticoagulation in light of coronary ectasia and likely in situ thrombosis.
embolization of thrombus from the mid-RCA (Figure 4B). With clinical resolution of the myocardial infarction, TIMI-3 flow in the vessel, and only mild focal stenosis of the mid-RCA, no invasive intervention was undertaken. The patient was managed on aspirin, IV unfractionated heparin, and clopidogrel while in hospital, and was discharged on aspirin and warfarin with the plan for indefinite systemic anticoagulation in light of coronary ectasia and likely in situ thrombosis.
Six months later, the patient elected to discontinue warfarin, and presented 1 week thereafter with recurrent inferior myocardial infarction. Coronary angiography revealed extensive thrombus in the mid-RCA with thrombus in the posterior descending artery and posterior lateral ventricular branches, likely reflective of recurrent in situ thrombosis with distal embolization (Figure 4C). He was treated with aspirin, clopidogrel, eptifibatide, and continuous IV unfractionated heparin infusion. Re-look angiography 5 days later revealed resolution of RCA thrombus (Figure 4D). He was discharged on aspirin and a LMWH bridge to therapeutic warfarin, with plan to continue systemic anticoagulation indefinitely. He has continued to do well throughout follow-up.
Case 5. A 56-year-old male with past medical history of gastroesophageal reflux disease and seasonal allergies presented with cardiogenic shock. Transthoracic echocardiogram at the time of admission was notable for biventricular heart failure with ejection fraction of 20% and severe mitral regurgitation. Coronary angiography was notable for diffuse CAA disease throughout both left and right coronary arteries. IVUS was performed and the left main was dilated to an estimated 20 mm (Figure 5). Cardiac magnetic resonance imaging with gadolinium demonstrated diffusely delayed gadolinium enhancement consistent with subacute inflammation. Myocardial biopsy demonstrated mild to moderate myocyte hypertrophy and multifocal interstitial fibrosis, consistent with dilated  cardiomyopathy without evidence of active myocarditis. The remainder of the serologic work-up did not yield an obvious etiology of the presenting cardiomyopathy. While the patient initially required intensive care unit level care, intra-aortic balloon counterpulsation, inotropic and pressor support, his clinical condition improved with supportive care. The patient gradually improved during follow-up and was discharged on life-long aspirin and clopidogrel for management of CAA in addition to a medical regimen for non-ischemic cardiomyopathy.
cardiomyopathy without evidence of active myocarditis. The remainder of the serologic work-up did not yield an obvious etiology of the presenting cardiomyopathy. While the patient initially required intensive care unit level care, intra-aortic balloon counterpulsation, inotropic and pressor support, his clinical condition improved with supportive care. The patient gradually improved during follow-up and was discharged on life-long aspirin and clopidogrel for management of CAA in addition to a medical regimen for non-ischemic cardiomyopathy.
Discussion
CAA was first described by Morgagni in 1761 and the first in vivo case was reported by Munker et al in 1958.3,4 While multiple lesion classifications exist to describe CAA, generally, the term aneurysm is used to describe a localized, abnormal dilatation of a coronary artery that is either saccular or fusiform in shape, while the term ectasia is used to describe diffuse dilatation.4 The term “giant” CAA is generally reserved for a dilation that exceeds the reference vessel diameter by 4 times.5
In the adult population, CAA is most commonly considered a variant of CAD accounting for 50% of documented cases.1 Swaye et al reviewed 20,087 patients in 1983 from the National Institutes of Health sponsored CASS registry and found an incidence of 978 cases of CAA (4.9%) among patients undergoing coronary angiography for suspected coronary disease.1 They found there were no significant differences between aneurysmal and non-aneurysmal coronary disease patients when features such as hypertension, diabetes, dyslipidemia, family history, cigarette consumption, incidence of documented myocardial infarction, presence and severity of angina, and presence of peripheral vascular disease were examined. The authors further noted there was no difference in 5-year survival between the patients with aneurysmal coronary disease and those with any amount of CAD. They concluded that aneurysmal CAD is not likely a distinct clinical entity but a variant of CAD.1 Other  etiologies for CAA include congenital (20%-30%), inflammatory or vasculitis (10%-20%), and connective tissue disorders (5%-10%).6 Iatrogenic etiology following attempted PCI is a rare (0.3%-0.6%) but important cause of CAA.7 CAA has been documented following PCI resulting from residual dissection and deep arterial wall injury after the use of oversized balloons or stents, high-pressure inflations, and atherectomy.7,8
etiologies for CAA include congenital (20%-30%), inflammatory or vasculitis (10%-20%), and connective tissue disorders (5%-10%).6 Iatrogenic etiology following attempted PCI is a rare (0.3%-0.6%) but important cause of CAA.7 CAA has been documented following PCI resulting from residual dissection and deep arterial wall injury after the use of oversized balloons or stents, high-pressure inflations, and atherectomy.7,8
Histopathologic findings in autopsy cases have also revealed extensive atherosclerotic changes with destruction or thinning of the media of the vessel wall in CAA similar to that seen in CAD.9 Matrix metalloproteinases (MMPs) have been implicated in the pathogenesis of CAA formation through increased proteolysis of extracellular matrix proteins.10 Some authors also postulate that heterogeneous depth of vascular injury caused by inflammation and atherosclerosis leads to varying degrees of remodeling within the coronary artery whereby aneurysmal segments may lie adjacent to segments of stenosis.11 CAA appears when the atheromatous process affects the intima, media, and adventitial parts of the vessel, which leads to remodeling and dilatation.12 CAA then progresses via Laplace’s law where increasing diameter leads to increasing wall stress, which in turn causes progressive dilatation of the affected arterial segment.13
Coronary aneurysm may promote thrombosis through abnormal flow conditions.13 Within the aneurysm itself there is relatively slower or static flow, which promotes platelet activation and thrombus formation.14 Additionally, in the instance of poststenotic dilatation, platelets may be activated by turbulent flow and shear stress through a more proximal stenosis. The combination of a proximal stenosis and an immediately adjacent region of slower coronary blood flow within an aneurysm represents a powerful stimulus promoting thrombus formation. Additionally, turbulent poststenotic flow within the coronary aneurysm likely promotes endothelial activation. Finally, the presence of chronic thrombosis within an aneurysm may also promote thrombogenesis by providing clotting precursors and fibrin as nidus for new clot.13 Hence, CAA thrombosis is mediated both from platelet and endothelial-derived pathophysiologic mechanisms and may be further propagated in the presence of chronic thrombus.13
Many clinical questions remain regarding the management of CAA, particularly in the setting of ACS. First, the chronicity of thrombus visualized angiographically within CAA may be difficult to discern. As highlighted in Case 2, the patient presented with ACS despite chronic systemic anticoagulation and antiplatelet therapy, making acute thrombosis less likely. It was thus unclear if the clinical presentation represented classic “plaque rupture” in ACS, or alternatively, embolization of chronic thrombus from CAA provoking myocardial injury. In a post mortem study, Daoud et al reported the presence of thrombus in 7 of 10 patients with CAA,15 suggesting that chronic thrombosis may be common.
Several case reports have highlighted various medical strategies that were successful in the management of individual patients with CAA thrombosis. Myler et al described a case of successful treatment of CAA with intracoronary urokinase, IV heparin, and oral anticoagulation in a young woman with CAA secondary to presumed Kawasaki disease.16 Other authors have documented angiographic thrombus resolution and excellent clinical outcomes with the use of intravenous eptifibatide, heparin, and aspirin and discharge with long-term dual-antiplatelet therapy.17 Lima et al reported two cases of left main CAA that were treated non-surgically with warfarin and aspirin who remained well at 6-month follow-up exam.18 To date, however, no peer-reviewed or consensus document addressing the acute or long-term medical management of CAA with ACS exists.
In cases of CAA where coronary ischemia persists despite medical optimization, surgical or percutaneous revascularization may be required. PCI of CAA, particularly in the instance of thrombosis, may represent several technical challenges. Two important potential complications include distal embolization of thrombus and stent malapposition. Yip et al reported no-reflow phenomenon (defined as ≤ TIMI-2 flow) and distal embolization after primary PCI in 68.2% and 70% in patients with visibly thrombosed CAA.19 As highlighted in Case 1, instrumenting a vessel with aneurysmal dilatation — in this case, the simple act of passing a coronary guidewire through the aneurysm — may precipitate distal embolization of associated thrombus. Placement of a stent within an aneurysmal segment poses a technical challenge, since apposition of stent struts to a vessel of large and irregularly-variable caliber may not be feasible. Leaving unapposed stent struts — whether bare metal or drug eluting — may represent a nidus for thrombosis.21 The use of PTFE-covered stents may also pose unique challenges: deployment of a covered stent in CAA may result in occlusion of branch arteries that originate within the subtended aneurysm; incomplete coverage of the aneurysm may result in persistent “leak” into the aneurysm sac; and, PTFE-covered coronary stents pose risk for thrombosis or in-stent restenosis.21,22 Stent length and aneurysm caliber (diameter >10 mm) have also been reported as independent risk factors for future restenosis with PTFE-covered stents.23
Despite these potential complications, there are many documented cases of successful percutaneous exclusion of CAA using PTFE-covered stent technique, such as in Case 3. Lee et al reported their experience with a patient with CAA who presented with ACS and was treated with AngioJet rheolytic thrombectomy (Medrad) followed by covered JoStent placement (Abbott Vascular).24 The authors suggest that exclusion of the aneurysm with a PTFE-covered stent graft would eliminate sluggish flow through the (previously) aneurysmal segment, and would reduce the likelihood of aneurysm thrombosis, enlargement, or future rupture. Follow-up angiography was not performed, but the patient remained asymptomatic at 1 year. Briguori et al described 8 patients with CAA treated successfully with PTFE-covered stents with 0% in-hospital major adverse cardiovascular events.21 Angiographic follow-up at 10 ± 6 months was performed in all patients, and was notable for in-stent restenosis in 12.5%, which required target lesion revascularization.21 At 35 ± 8 months, 6 patients were symptom free.21 Bare-metal stent (BMS) implantations have also been used successfully in the treatment of CAA, yet are often used when PTFE-covered stent delivery is unsuccessful. Iakovou et al described 3 such cases where CAAs were treated with BMS.20 They observed immediate stagnation of contrast within the space between stent and aneurysm wall; at 10 ± 6 months of angiographic follow-up, all aneurysms had been completely abolished. However, 2 of the lesions had in-stent restenosis requiring placement of an additional drug-eluting stent (DES).20 There are also rare case reports of successful hybrid interventions using both PTFE-covered stent and BMS involving CAAs with bifurcation lesions and tapering CAAs.25,26
Other authors have documented successful treatment of CAA using coil embolization. Saccá et al described a case of successful coil embolization and occlusion of CAA in the distal left main coronary in a patient with prior coronary artery bypass graft (CABG) surgery, including left internal mammary artery to LAD.28 The authors successfully deployed four Guglielmi detachable coils (Boston Scientific) into the aneurysm and confirmed complete resolution of the aneurysm and patent native left main at final angiography.
Surgery may be indicated in the presence of aneurysms three to four times the original vessel diameter (giant CAA), involvement of the left main, bifurcation lesions, or multivessel involvement.29 Surgical treatment entails coronary artery bypass with or without aneurysm ligation or resection.2 In a retrospective analysis comparing surgery and PTFE-covered stents for the management of CAA, Szalat et al reported no procedural deaths were reported in either group.23 There are several case reports of successful surgical treatment of CAA.28,29 No short-term survival difference in patients with CAA who underwent CABG compared to controls was seen, however, and long-term survival difference remains unknown.30 Some authors have suggested the indications for surgical management of CAA are the following: (1) CAA near bifurcation of large branches; (2) evidence of emboli from the aneurysm to the distal coronary bed resulting in myocardial ischemia; (3) progressive enlargement of a CAA documented by serial angiographic measurements; and (4) CAAs in the left main stem.15 Other authors also included the presence of a symptomatic giant CAA as an indication for surgery.31 While there are several reported cases of successful surgical repair of CAA in adults,31,32 some attempts at excision or plication of the coronary artery aneurysm in children with Kawasaki disease have not been successful and have resulted in death.13
Proposed Management Recommendations
To date, no consensus document or guidelines exist regarding the management of CAA in the setting of ACS for adults. Since the coincidence of CAA and ACS is a rare event, a prospective, randomized clinical trial to compare pharmacologic and/or invasive management strategies is not likely logistically feasible. By combining the perspective derived from a review of the available clinical literature, extrapolating from American Heart Association (AHA) pediatric recommendations regarding CAA thrombosis in Kawasaki disease,13 and applying current American College of Cardiology (ACC)/AHA guidelines for management of ACS,33 we propose the clinical management strategies outlined below and in Figure 6. These strategies are not intended to replace ACC/AHA clinical guidelines for ACS and are not endorsed by the ACC, AHA, European Society of Cardiology, or Society of Thoracic Surgeons. Ultimately, management decisions should always be tailored to the individual patient, and should include a frank discussion between the patient and the medical care team reviewing the risk/benefit of all potential clinical options, highlighting patient preference, consulting physician recommendations, local expertise with various treatment options, and AHA/ACC ACS guidelines.33
The following recommendations are intended for management of patients presenting with ACS where CAA is discovered on diagnostic coronary angiography and the aneurysmal artery is suspected to be culprit for the clinical syndrome (Figure 6).
1. Initial Management: Antiplatelet and Anticoagulant Therapy
Antiplatelet therapy should be initiated immediately upon the identification of CAA with ACS if not previously administered.33 Aspirin 162 or 325 mg daily is preferred; and a second antiplatelet agent (such as clopidogrel, prasugrel, or ticagrelor) should be strongly considered unless there is a clinical contraindication. Anticoagulation with intravenous weight-based unfractionated heparin (UFH) or subcutaneous LMWH should be added to antiplatelet therapy. If copious thrombus is noted within CAA during angiography, we recommend additional consideration of glycoprotein IIb/IIIa inhibitor infusion for 24-48 hours. There is greater experience with such longer-term infusions of eptifibatide than with abciximab; we therefore recommend eptifibatide for this application. Glycoprotein IIb/IIIa infusion should be accompanied by close monitoring for thrombocytopenia, anemia, or bleeding.
2. Conservative Versus Invasive Strategy
Most patients presenting with ACS in the context of culprit CAA identified at coronary angiography should be managed conservatively with antiplatelet and antithrombotic therapies. Similar to the AHA/ACC guidelines for the management of patients with unstable angina and non-ST segment elevation myocardial infarction,33 we recommend that patients with the following be considered for revascularization:
- TIMI 0 or 1 flow in the aneurysmal vessel;
- Patients with recurrent angina or ischemia;
- Sustained ventricular tachycardia; or
- Hemodynamic instability including sustained hypotension.
If these findings are not present during the initial clinical assessment and diagnostic cardiac catheterization, we recommend a conservative management strategy including dual-antiplatelet therapy, anticoagulation with heparin (UFH or LMWH), and consideration for IV glycoprotein IIb/IIIa for 24-48 hours, particularly in the context of significant, angiographically-evident thrombus. Regardless of initial management strategy, all patients with CAA and ACS should be admitted for continuous cardiac monitoring. Patients who are triaged to a conservative strategy who develop any of the following should be referred for repeat coronary angiography and possible revascularization:
- TIMI 0 or 1 flow on repeat angiography;
- Recurrent angina or ischemia;
- Sustained ventricular tachycardia;
- Hemodynamic instability;
- Angina at rest or with low-level activities despite intensive anti-ischemic therapy; or
- Congestive heart failure symptoms, an S3 gallop, pulmonary edema, worsening rales.
Invasive Strategy: Percutaneous Versus Surgical Revascularization
If revascularization is indicated, it is often valuable to involve specialists in endovascular and surgical specialties to provide balanced insight into optimal revascularization for CAA. If endovascular therapy is pursued, great care should be taken to avoid thrombus embolization during instrumentation of the CAA: a soft-tipped coronary guidewire should be manipulated meticulously through the CAA, taking care not to coil the wire tip in the body of the aneurysm; distal embolic protection could be considered, particularly in the context of copious thrombus; aspiration thrombectomy is often necessary to reduce thrombus burden and improve coronary flow. The use of IVUS or optical coherence tomography (OCT) may further define the lesion characteristics including vessel diameter, presence of thrombus, and relationship of the CAA to branch vessels.
From our review of the available literature and ACC/AHA ACS guidelines, we propose that patients with the following findings be referred for surgical revascularization:
- CAA involving the left main coronary artery;
- Multivessel CAD;
- Giant CAA (dilation that exceeds the reference vessel diameter by 4 times);
- CAA involving bifurcation of significant side-branch vessel; or
- Other separate indications for cardiothoracic surgery unrelated to CAA.
If these findings are not present or the patient is not a surgical candidate, percutaneous intervention for CAA is a reasonable management strategy. The best choice of interventional treatment for CAA is left to the local expertise of the interventional cardiologist and the unique findings on coronary angiography. We would suggest PTFE-covered stents as first-line therapy in CAA if they can be delivered across the lesion. PTFE-covered stents may be less flexible, making their delivery in tortuous or heavily calcified vessels difficult. However, if they are successfully delivered, they can theoretically seal the aneurysm, thereby preventing the risk of rupture or distal embolization of debris.21,24,34 If PTFE-coated stent delivery is unsuccessful, it is reasonable to consider treatment of CAA with DES or BMS implantation. While there is a paucity of literature on this topic, available case reports suggest that the stent scaffolding promotes stagnation of blood within the CAA and promotes aneurysm occlusion.20 The choice of stent (BMS vs DES) should be determined by the interventional cardiologist and the pertinent clinical details unique to each case. While there were initial reports of first-generation DES implantation having a higher incidence of associated late CAA compared to BMS (1.4% vs 0.2%), there are limited data regarding the current generation of DESs.35 Finally, if BMS or DES cannot be delivered across the lesion, it is reasonable to consider salvage angioplasty or surgical revascularization.
Discharge Antiplatelet and Anticoagulation Strategies
In patients with giant CAA or with other indications for chronic systemic anticoagulation, we recommend chronic therapy with aspirin 81 mg daily and warfarin to target an international normalized ratio (INR) of 2.0-3.0.13 In the majority of other cases, however, we recommend dual-antiplatelet therapy with aspirin 81 mg daily and clopidogrel, prasugrel, or ticagrelor, regardless of whether conservative or invasive strategy is pursued.33,36 The duration of dual-antiplatelet therapy in patients presenting with ACS and CAA is unclear, and should be tailored to the patient, lesion, and treatment approach. The duration of dual-antiplatelet therapy after PTFE stenting is also unclear, but physicians may consider prolonged dual-antiplatelet therapy given evidence of delayed endothelialization and increased rates of subacute stent thrombosis.37,38 If a patient is readmitted with ACS while on dual-antiplatelet therapy and an embolic source from the aneurysm is thought to be the culprit, anticoagulation with INR-adjusted warfarin (2.0-2.5) in combination with aspirin 81 mg daily should be considered if a conservative management strategy is planned. The role of the novel oral anticoagulants for treatment of CAA is unknown at this time and is not likely to be studied given the paucity of patients with CAA and ACS. Off-label use may be considered following discussion with the patient, including careful consideration of potential risks and benefits.
Conclusion
CAA with ACS is an uncommon clinical finding; its management poses several unique challenges. The cases presented in this manuscript demonstrate successful — and distinct — strategies for the management of CAA with ACS, but also highlight some of the possible complications. We present an algorithm for management of CAA with ACS, based on extensive review of the literature and pertinent clinical guidelines. Armed with this perspective, multidisciplinary engagement, and a focus on patient and lesion-specific factors, we may provide tailored care to optimize patient outcomes in this uncommon clinical scenario.
References
- Swaye PS, Fisher LD, Litwin P, et al. Aneurysmal coronary artery disease. Circulation. 1983;67(1):134-138.
- Syed M, Lesch M. Coronary artery aneurysm: a review. Prog Cardiovasc Dis. 1997;40(1):77-84.
- Morgagni JB. De Sedibus et Causis morborum. In: Venectus, Tom I. 1761;Epis 27(Art 28).
- Munker TM, Peterson O, Vesterdal J. Congenital aneurysm of the coronary artery with an arteriovenous fistula. Acta Radiol. 1958;150:333-336.
- Kato H, Sugimura T, Akagi T, et al. Long-term consequences of Kawasaki disease. A 10 to 21-year follow-up study of 594 patients. Circulation. 1996;94(6):1379-1385.
- Robinowitz M, Forman MB, Virmani R. Nonatherosclerotic coronary aneurysms. In: Virmani R, Forman MB (eds). Nonatherosclerotic Ischemic Heart Disease. New York: Raven Press, 1989.
- Bell MR, Garratt KN, Bresnahan JF, et al. Relation of deep arterial resection and coronary artery aneurysms after directional coronary atherectomy. J Am Coll Cardiol. 1992;20(7):1474-1481.
- Slota PA, Fischman DL, Savage MP, et al. Frequency and outcome of development of coronary artery aneurysm after intracoronary stent placement and angioplasty. STRESS trial investigators. Am J Cardiol. 1997;79(8):1104-1106.
- Turhan H, Erbay AR, Yasar AS, et al. Plasma soluble adhesion molecules intervessicular adhesion molecule-1, vascular cell adhesion molecule-1 and E-selectin levels in patients with isolated coronary artery ectasia. Coron Artery Dis. 2005;16(1):45-51.
- Newman KM, Ogata Y, Malon AM, et al. Identification of matrix metalloproteinases 3 (stromelysin-1) and 9 (gelatinase B) in abdominal aortic aneurysm. Arterioscler Thromb. 1994;14(8):1315-1320.
- Pahlavan P, Niroomand, F. Coronary artery aneurysm: a review. Clin Cardiol. 2006;29(10):439-443.
- Markis JE, Joffe CD, Cohn, PF, Feen DJ, Herman MV, Gorlin R. Clinical significance of coronary arterial ectasia. Am J Cardiol. 1976;37(2):217-222.
- Newburger JW, Takahashi M, Gerber MA, et al; Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747-2771.
- Swanton HR, Thomas ML, Coltart DJ, et al. Coronary artery ectasia: a variant of occlusive coronary arteriosclerosis. Br Heart J. 1978;40(4):393-400.
- Daoud A, Pankin D, Tulgan H, Florentin R. Aneurysms of the coronary artery: report of ten cases and review of the literature. Am J Cardiol. 1963;11:228-237.
- Myler R, Schechtmann N, Rosenblum J, et al. Multiple coronary artery aneurysms in an adult associated with extensive thrombus formation resulting in acute myocardial infarction: successful treatment with intracoronary urokinase, intravenous heparin, and oral anticoagulation. Catheter Cardiovasc Diagn. 1991;24(1):51-54.
- Vik-Mo H, Wiseth R, Hegbim K. Coronary aneurysm after implantation of paclitaxel-eluting stent. Scand Cardiovasc J. 2004;38(6):349-352.
- Lima B, Varma S, Lowe J. Nonsurgical management of left main coronary artery aneurysms. Tex Heart Inst J. 2006;33(3):376-379.
- Yip H-K, Chen M-C, Wu C-J, et al. Clinical features and outcome of coronary artery aneurysm in patients with acute myocardial infarction undergoing a primary percutaneous coronary intervention. Cardiology. 2002;98(3):132-140.
- Iakovou, I, Dimopulos A, Dangas G. Normal to normal: a method of treatment of coronary aneurysms with deployment of bare-metal stents. J Invasive Cardiol. 2011;23(5):E121-E125.
- Briguori C, Sarais C, Sivieri G, Takagi T, Di Mario C, Colombo A. Polytetrafluoroethylene-covered stent and coronary artery aneurysms. Catheter Cardiovasc Interv. 2002;55(3):326-330.
- Lampropoulos M, Tsotsoros N, Aggeli C, Sokolis D, Iliopoulos T, Stefanadis C. Diagnostic approach in symptomatic or asymptomatic aneurysmal disease of the coronary arteries: a case report and five-year angiographic follow-up. Hellenic J Cardiol. 2011;52(4):357-360.
- Szalat A, Durst R, Cohen A, et al. Use of polytetrafluoroethylene-covered stent for treatment of coronary artery aneurysm. Catheter Cardiovasc Interv. 2005;66(2):203-208.
- Lee M, Nero T, Makkar R, Wilent J. Treatment of coronary aneurysm in acute myocardial infarction with AngioJet thrombectomy and JoStent coronary stent graft. J Invasive Cardiol. 2004;16(5):294-296.
- Orlic D, Vitrella G, Corvaja N, Colombo A. New technique to seal a long giant coronary aneurysm with PTFE-covered stents: a case report. Catheter Cardiovasc Interv. 2006;67(1):41-45.
- Iakovou I, Colombo A. Treatment of a coronary aneurysm involving bifurcation with the use of a custom-made polytetrafluoroethylene-covered bifurcation stent system. Catheter Cardiovasc Interv. 2005;64(2):169-172.
- Zeb M, McKenzie DB, Scott PA, Talwar S. Treatment of coronary aneurysms with covered stents: a review with illustrated case. J Invasive Cardiol. 2012;24(9):465-469.
- Saccá S, Pacchioni A, Nikas D. Coil embolization for distal left main aneurysm: a new approach to coronary artery aneurysm treatment. Catheter Cardiovasc Interv. 2012;79(6):1000-1003.
- Chia HM, Tan KH, Jackson G. Non-atherosclerotic coronary artery aneurysms: two case reports. Heart. 1997;78(6):613-616.
- Aintablian A, Hamby RI, Hoffman, Kramer RJ. Coronary ectasia: incidence and results of coronary bypass surgery. Am Heart J. 1978;96(3):309-315.
- Li D, Sun L, Song Y, et al. Surgical treatment of giant coronary artery aneurysm. J Thorac Cardiovasc Surg. 2005;130(3):817-821.
- Okada Y, Doi K, Meshii K, Kono S. Surgical treatment in an adult with coronary artery aneurysm at proximal site of left anterior descending artery: report of a case. Kyobu Geka. 2011;64(10):933-935.
- Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina). Circulation. 2000;102(10):1193-209. Erratum in: Circulation. 2000;102(14):1739.
- Gercken U, Lansky AJ, Buellesfeld L, et al. Results of the Jostent coronary stent graft implantation in various clinical settings: procedural and follow-up results. Catheter Cardiovasc Interv. 2002;56(3):353-360.
- Stone GW, Ellis SG, Cannon L, et al; for the TAXUS V investigators. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005;294(10):1215-1223.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494-502. Errata in: N Engl J Med. 2001;345(20):1506 and N Engl J Med. 2001;345(23):1716.
- Takano M, Yamamoto M, Inami T, Murakami D, Seino Y, Mizuno K. Delayed healing of a coronary stent graft. JACC Cardiovasc Interv. 2011;4(4):466-467.
- Takano M, Yamamoto M, Inami S, et al. Delayed endothelialization after polytetrafluoroethylene-covered stent implantation for coronary aneurysm. Circ J. 2009;73(1):190-193.
_________________________________
From the 1Division of Cardiology, University of California, San Francisco, San Francisco, California and 2Cardiovascular Division, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, 3School of Medicine, University of Iowa Hospitals and Clinics, Iowa City, Iowa, and 4Division of Cardiology, Department of Internal Medicine, University of New Mexico, Albuquerque, New Mexico.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Drachman reports research grant support from Atrium Medical Corporation, Baxter Healthcare Corporation, iDev Technologies, Inc, Lutonix/Bard; Clinical Events Committee for PLC Medical Systems, Inc; and Data and Safety Monitoring Board for Prairie Education & Research Cooperative. The remaining authors report no conflicts of interest regarding the content herein.
Manuscript submitted December 11, 2013 and accepted December 19, 2013.
Address for correspondence: Nathan Boyer, MD, 505 Parnassus Ave, Box 0103, San Francisco, CA 94143-0124. Email: nathan.boyer@ucsf.edu














