Comparison of Twelve-Month Outcomes After Percutanous Coronary Intervention With Everolimus-Eluting Versus Zotarolimus-Eluting or Sirolimus-Eluting Stents From the PROENCY (PROmus ENdeavor CYpher) Registry
Abstract: Objectives. We compared safety and efficacy outcomes of 3 limus-based drug-eluting stents in the ‘all-comers’ PROENCY (PROmus/ENdeavor /CYpher) registry. Background. Limited data are available on head-to-head comparisons of the everolimus-eluting stent (EES) with the zotarolimus-eluting stent (ZES) or the sirolimus-eluting stent (SES) in the treatment of patients with coronary artery disease. Methods. PROENCY was a prospective, open-label, multicenter, observational study including consecutive patients undergoing planned treatment with EES, ZES, or SES. Seventeen centers were designated to place an EES or SES, 14 other centers were designated to place EES or ZES. The primary endpoint was the composite of cardiac death, myocardial infarction, and target vessel revascularization (TVR) at 12 months. Unadjusted and propensity-adjusted outcomes were compared between groups. Results. A total of 1921 patients were enrolled in the study from February to December 2008, of which 1704 patients received only study stents and were analyzed. At 12 months, the unadjusted major adverse event rate was significantly lower in the EES group versus the ZES group (3.1% vs 8.7%; P=.001) and the SES group (5.2% vs 9.6%; P=.01). This was mainly driven by lower TVR rates [2.6% with EES vs 8.2% with ZES [P<.001] and 4.1% with EES vs 7.0% with SES [P=.05]. Stent thrombosis rates were low and comparable. Adjusted analyses confirmed the unadjusted results. Conclusion. There were no differences in safety outcomes of EES, ZES, and SES at 12 months in PROENCY. However, differences in efficacy were observed between the 3 “limus”-based stents in a real-world patient population.
J INVASIVE CARDIOL 2012;24(10):495-502
Key words: PROENCY, zotarolimus-eluting stent, everolimus-eluting stent, sirolimus-eluting stent
___________________________________________________________
Drug-eluting stent (DES) use has reduced the incidence of repeat revascularization when compared with bare-metal stent (BMS) use.1-3 This reduction is achieved by the slow release of antiproliferative and anti-inflammatory drugs, which are coupled to the metal stent platform by a polymer. Currently, agents from the “limus” family of antiproliferative drugs, such as sirolimus and its analogues zotarolimus and everolimus, are predominantly used in contemporary DESs. These rapamycin derivates inhibit cytokine and growth-factor mediated proliferation of lymphocytes and smooth muscle cells, resulting in reduced in-stent neointimal proliferation.4 The occurrence of stent thrombosis (ST) has emerged as one of the safety issues with DESs.5 The use of the first-generation sirolimus-eluting stent (SES) is associated with an increased risk of very late stent thrombosis.6 Compared with the SES, the second-generation zotarolimus-eluting stent (ZES) and everolimus-eluting stent (EES) are characterized by thinner stent struts, a more biocompatible polymer, and newer “limus” analogues.4-7 Consequently, second-generation DESs are potentially more efficacious and safer than first-generation DESs.
Regarding direct comparisons of “limus” stents, ZESs have been compared with SESs in the randomized ENDEAVOR III, SORT OUT III, and ZEST trials, indicating a better performance with SES.8-11 Comparisons of SES or ZES with EES are sparse, or based on a new version of the ZES.12 Therefore, the PROmus, ENdeavor, CYpher (PROENCY) registry was designed to collect data on the use and 1-year clinical outcomes of the second-generation EES (Promus; Boston Scientific), ZES (Endeavor; Medtronic CardioVascular), and first-generation SES (Cypher; Cordis Corporation) in a multicenter “real world” setting. In the current study, we present the 12-month clinical outcomes of the PROENCY registry. Moreover, we investigated the preference criteria of operators who have the choice between different “limus” stents.
Methods
 Study design. PROENCY was a prospective, open-label, observational multicenter registry. A total of 1921 consecutive patients were included between February and December 2008 from 32 centers in Germany and France (Appendix 1). The objective of the PROENCY registry was to collect real-life outcomes on the everolimus-eluting Promus stent, sirolimus-eluting Cypher stent, and zotarolimus-eluting Endeavor stents in routine clinical practice. All stents have received the CE mark and are commonly used in Europe. Consecutive patients were included in a 1:1 distribution of the EES with the comparator SES in EES/SES centers, and in a 1:1 distribution of the EES with the comparator ZES in EES/ZES centers. Participating centers determined whether to join the SES or ZES comparator group. The selection of stent type was according to the physician’s preferred choice and routine clinical practice. The study was conducted according to the Declaration of Helsinki, and written informed consent was obtained.
Study design. PROENCY was a prospective, open-label, observational multicenter registry. A total of 1921 consecutive patients were included between February and December 2008 from 32 centers in Germany and France (Appendix 1). The objective of the PROENCY registry was to collect real-life outcomes on the everolimus-eluting Promus stent, sirolimus-eluting Cypher stent, and zotarolimus-eluting Endeavor stents in routine clinical practice. All stents have received the CE mark and are commonly used in Europe. Consecutive patients were included in a 1:1 distribution of the EES with the comparator SES in EES/SES centers, and in a 1:1 distribution of the EES with the comparator ZES in EES/ZES centers. Participating centers determined whether to join the SES or ZES comparator group. The selection of stent type was according to the physician’s preferred choice and routine clinical practice. The study was conducted according to the Declaration of Helsinki, and written informed consent was obtained.
Patients and procedures. Patients undergoing percutaneous coronary intervention (PCI) with at least one lesion suitable for stenting with one of the study stents in accordance with the instructions for use were considered eligible for the PROENCY registry. There were no clinical or angiographic exclusion criteria to allow for an all-comers design. The use of dual-antiplatelet therapy and other concomitant medication was according to standard medical practice.
Data collection and management. Baseline patient and lesion, procedure-related, and angiographic characteristics were collected and stored in a central internet-based electronic data capture system (Eventa; KIKA Medical). All events were assessed at 6 and 12 months post procedure using scripted telephone interviews or during an outpatient clinic visit according to standard local practice. All site personnel were trained on the protocol and internet-based database prior to the initiation of the registry at each center. During the enrollment period, baseline clinical and lesion characteristics for the respective registry stent groups were regularly reviewed to observe any trends toward a subject selection bias. Operators were blinded to this procedure. All patients who had reported events and an additional 10% of patients without events were monitored with full-source data verification by an external medical monitoring committee (EMMC). Ten percent of the sites were selected randomly for on-site monitoring including full-source data verification.
Outcomes. The primary outcome of the PROENCY registry was the patient-oriented hierarchical composite of cardiac death, any myocardial infarction (MI), and target vessel revascularization (TVR) at 12 months post index procedure and its individual components. Secondary outcomes included the stent-oriented hierarchical composite of stent-related cardiac death, target vessel-related MI, and target lesion revascularization (TLR) at 12 months and its individual components. Other secondary outcomes include stent thrombosis at 6 and 12 months, 6-month outcomes, technical success, and stent deliverability. Cardiac death was defined as all-cause death, unless an unequivocal non-cardiac cause could be established. The outcome MI was defined as one of the following criteria: an elevation in CK above 2 times the upper limit of normal (ULN) with a positive CK-MB, CK above 5 times the ULN with a positive CK-MB after coronary artery bypass grafting (CABG), a troponin level above the ULN, or electrocardiographic evidence of new pathologic Q-waves in at least 2 contiguous leads with positive CK-MB. The outcome TVR was defined as any percutaneous or surgical re-intervention of a target vessel, and TLR as a re-intervention of the target lesion. Stent thrombosis was considered as definite or probable according to the Academic Research Consortium (ARC) definition.13 Finally, technical success was defined as successful delivery and deployment of the registry stents, and stent deliverability as the index procedure duration. All major cardiac events were adjudicated by the EMMC.
Statistical analysis. Results are reported according to the EES/SES arm or EES/ZES arm, the same EES patients are not used for comparison with SES or ZES. Categorical variables were reported as counts and percentages, and continuous variables as means and standard deviations (SD) or median and interquartile ranges (IQR). Cumulative event rates were estimated using the Kaplan-Meier method and compared with the log-rank test. Hazard ratios were calculated with Cox proportional-hazards models. Follow-up was censored at the last known date of follow-up or at 12 months, whichever came first. A P-value <.05 was deemed significant.
To adjust for the non-randomized design of the PROENCY registry, a propensity score 1:1 matching analysis was performed. The propensity score model was based on the following variables: gender, age, prior PCI, prior CABG, prior MI, hypertension, insulin-requiring diabetes mellitus, hyperlipidemia, renal disease, multivessel disease, reference vessel diameter, lesion length, left anterior descending artery located lesion, American College of Cardiology/American Heart Association lesion type B2/C, and target lesion diameter stenosis. Statistical analyses were performed by Biostatisticians at Boston Scientific Corporation and supervised by the Academic Medical Center – University of Amsterdam.
Results
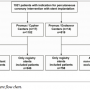 Patients and procedures. A flowchart of the 1921 patients included in the PROENCY registry is shown in Figure 1. In the EES/SES centers, a total of 1102 patients were included while 819 patients were included in the EES/ZES centers. Patients exclusively treated with registry stents included 946 in the EES/SES arm and 758 in the EES/ZES arm and comprise the final analysis cohort. The baseline characteristics of the patients in the EES/SES and EES/ZES arms are presented in Table 1, procedural characteristics are shown in Table 2. In the
Patients and procedures. A flowchart of the 1921 patients included in the PROENCY registry is shown in Figure 1. In the EES/SES centers, a total of 1102 patients were included while 819 patients were included in the EES/ZES centers. Patients exclusively treated with registry stents included 946 in the EES/SES arm and 758 in the EES/ZES arm and comprise the final analysis cohort. The baseline characteristics of the patients in the EES/SES and EES/ZES arms are presented in Table 1, procedural characteristics are shown in Table 2. In the  EES/SES arm, patients who received an EES more often had hypercholesterolemia, insulin-dependent diabetes mellitus, and multivessel disease. More patients in the EES group received multiple stents, while significantly fewer patients were treated for long (>28 mm; P=.005) or small diameter lesions (<2.5 mm; P<.001).
EES/SES arm, patients who received an EES more often had hypercholesterolemia, insulin-dependent diabetes mellitus, and multivessel disease. More patients in the EES group received multiple stents, while significantly fewer patients were treated for long (>28 mm; P=.005) or small diameter lesions (<2.5 mm; P<.001).
Patients in the EES/ZES arm were generally comparable, although patients treated with an EES more often had a history of previous PCI. Significantly fewer patients were treated with an EES for small diameter lesions (<2.5 mm; P=.006), but were treated more frequently for an in-stent restenosis (8.6% vs 4.4% in the ZES group; P=.009).
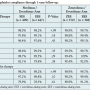
 Outcomes. In the EES/SES arm, the 12-month primary outcome of cardiac death, any MI, or TVR occurred in 9.6% of the SES-treated patients compared with 5.2% in the EES-treated patients (P=.01). This was mainly driven by numerically lower cardiac death (P=.07) and TVR (P=.05) in the EES-treated patients. The stent-oriented composite outcome of stent-related cardiac death, target vessel MI, or TLR occurred in 5.5% of the SES group compared with 2.5% of the EES group (P=.02). TLR rates were respectively 5.3% and 2.3% (P=.02), and no significant differences occurred in stent-related cardiac death or MI. Stent thrombosis rates were low and comparable between EES-treated (0.5%) and SES-treated (1.0%) patients (P=.31). Finally, technical success was comparable (99.1% with EES vs 100% with SES; P=.05) and stent deliverability, defined as the mean index procedure duration, was 38 minutes with EES vs 35 minutes with SES (P=.05). After propensity matching, 374 matched pairs were analyzed. The area under the receiver-operating characteristic curve (AUC) for this model was 0.61. Generally, the unadjusted clinical outcomes were confirmed. In the EES/SES arm, TLR rates were 5.4% in the SES group vs 2.3% in the EES group (P=.01).
Outcomes. In the EES/SES arm, the 12-month primary outcome of cardiac death, any MI, or TVR occurred in 9.6% of the SES-treated patients compared with 5.2% in the EES-treated patients (P=.01). This was mainly driven by numerically lower cardiac death (P=.07) and TVR (P=.05) in the EES-treated patients. The stent-oriented composite outcome of stent-related cardiac death, target vessel MI, or TLR occurred in 5.5% of the SES group compared with 2.5% of the EES group (P=.02). TLR rates were respectively 5.3% and 2.3% (P=.02), and no significant differences occurred in stent-related cardiac death or MI. Stent thrombosis rates were low and comparable between EES-treated (0.5%) and SES-treated (1.0%) patients (P=.31). Finally, technical success was comparable (99.1% with EES vs 100% with SES; P=.05) and stent deliverability, defined as the mean index procedure duration, was 38 minutes with EES vs 35 minutes with SES (P=.05). After propensity matching, 374 matched pairs were analyzed. The area under the receiver-operating characteristic curve (AUC) for this model was 0.61. Generally, the unadjusted clinical outcomes were confirmed. In the EES/SES arm, TLR rates were 5.4% in the SES group vs 2.3% in the EES group (P=.01).
 In the EES/ZES arm, the 12-month primary outcome of cardiac death, any MI, or TVR occurred in 8.7% of the ZES-treated patients vs 3.1% in the EES-treated patients (P=.001). TVR was significantly higher in the ZES group compared with the EES group (8.2% vs 2.6%, respectively; P<.001). The stent-oriented composite outcome of stent-related cardiac death, target vessel MI, or TLR occurred in 8.4% of the ZES group compared with 1.6% of the EES group (P<.001). Regarding the individual components, there was a trend toward higher stent-related cardiac death in the ZES group (P=.12) and a significantly higher TLR rate (7.9% vs 1.6% in the EES group; P<.001). Stent thrombosis rates were low and comparable between EES-treated patients (0.0%) and ZES-treated patients (0.5%; P=.31). Finally, technical success was comparable (99.2% with EES vs 99.7% with ZES; P=.62) and the mean index procedure duration was 46 minutes with EES vs 49 minutes with ZES (P=.26). After propensity matching, 320 matched pairs were analyzed in the EES/ZES arm. The AUC for this model was 0.59. The unadjusted clinical outcomes were also confirmed, and TLR rates were 8.2% in the ZES group vs 1.9% in the EES group (P<.001).
In the EES/ZES arm, the 12-month primary outcome of cardiac death, any MI, or TVR occurred in 8.7% of the ZES-treated patients vs 3.1% in the EES-treated patients (P=.001). TVR was significantly higher in the ZES group compared with the EES group (8.2% vs 2.6%, respectively; P<.001). The stent-oriented composite outcome of stent-related cardiac death, target vessel MI, or TLR occurred in 8.4% of the ZES group compared with 1.6% of the EES group (P<.001). Regarding the individual components, there was a trend toward higher stent-related cardiac death in the ZES group (P=.12) and a significantly higher TLR rate (7.9% vs 1.6% in the EES group; P<.001). Stent thrombosis rates were low and comparable between EES-treated patients (0.0%) and ZES-treated patients (0.5%; P=.31). Finally, technical success was comparable (99.2% with EES vs 99.7% with ZES; P=.62) and the mean index procedure duration was 46 minutes with EES vs 49 minutes with ZES (P=.26). After propensity matching, 320 matched pairs were analyzed in the EES/ZES arm. The AUC for this model was 0.59. The unadjusted clinical outcomes were also confirmed, and TLR rates were 8.2% in the ZES group vs 1.9% in the EES group (P<.001).
 All outcomes, including 6-month outcomes, are presented in Table 3. Kaplan-Meier curves for the patient-oriented composite outcome and TLR are shown in Figure 2. The clinical outcomes after propensity matching are presented in Table 4.
All outcomes, including 6-month outcomes, are presented in Table 3. Kaplan-Meier curves for the patient-oriented composite outcome and TLR are shown in Figure 2. The clinical outcomes after propensity matching are presented in Table 4.
Antiplatelet therapy. No differences were observed in aspirin, clopidogrel or dual-antiplatelet therapy compliance between EES and SES or EES and ZES patients through 1-year follow-up (Table 5).
Discussion
 PROENCY is a real-world all-comers registry that describes characteristics and outcomes of EES versus SES placement and EES versus ZES placement in a large cohort of consecutive patients undergoing contemporary PCI in Germany and France. Several implications can be drawn from the current analysis. First, operators who chose between SES and EES preferably chose EES in patients with insulin-requiring diabetes mellitus, multivessel disease or known hypercholesterolemia, and SES in case of long lesions, small vessels, and lesions located in the left anterior descending coronary artery. In ZES/EES centers, EES was preferably used in patients with a history of PCI and in restenotic lesions. Second, the 12-month outcomes show that the patient-oriented composite of cardiac death, MI, or TVR and the stent-oriented composite of stent-related cardiac death, target-vessel MI, or TLR were lower with EES placement compared with SES or ZES. This was mainly driven by reducing repeat revascularizations. Finally, after propensity matching, unadjusted clinical outcomes were confirmed.
PROENCY is a real-world all-comers registry that describes characteristics and outcomes of EES versus SES placement and EES versus ZES placement in a large cohort of consecutive patients undergoing contemporary PCI in Germany and France. Several implications can be drawn from the current analysis. First, operators who chose between SES and EES preferably chose EES in patients with insulin-requiring diabetes mellitus, multivessel disease or known hypercholesterolemia, and SES in case of long lesions, small vessels, and lesions located in the left anterior descending coronary artery. In ZES/EES centers, EES was preferably used in patients with a history of PCI and in restenotic lesions. Second, the 12-month outcomes show that the patient-oriented composite of cardiac death, MI, or TVR and the stent-oriented composite of stent-related cardiac death, target-vessel MI, or TLR were lower with EES placement compared with SES or ZES. This was mainly driven by reducing repeat revascularizations. Finally, after propensity matching, unadjusted clinical outcomes were confirmed.
 Previous studies. This is one of the few studies comparing EES with SES or ZES. In a recently published study, SES and EES were compared with BMS in large coronary arteries defined as a diameter of > 3 mm.3 TVR rates for reasons unrelated to MI were comparable among patients receiving SES (3.7%) and EES (3.1%). Although trial and registry results may not be directly comparable, and the trial was performed in a population at lower risk of restenosis because of the vessel diameter, the TVR rate in the SES group was lower and the TVR rate in the EES group was higher than those observed in our analysis. Results of LESSON I (Long-term comparison of Everolimus-eluting and Sirolimus-eluting Stents for cOronary revascularisatioN) were recently presented.14 In this propensity-matched analysis of 1342 patient pairs, the use of EES compared with SES was associated with a strong trend toward a lower composite of death, MI, or TVR (14.9% vs 18%; P=.056) and significantly lower MI (3.3% vs 5.0%; P=.017) and TVR rates (7.0% vs 9.6%; P=.039) at 3-year follow-up (median, 1.3 years). Moreover, SES versus EES comparisons have been reported from ISAR TEST IV, Efficacy of Xience/Promus versus Cypher to rEduce Late Loss in stENT (EXCELLENT) and SORT OUT IV.15 In the ISAR TEST IV trial, comparable outcomes were observed at 2-year follow-up. The composite of cardiac death, target vessel MI, or TLR occurred in 16.0% in the EES-treated patients vs 18.8% in the SES-treated patients (P=.23). The TLR rates were 9.9% versus 13.5% (P=.06), respectively. SORT OUT IV showed comparable clinical outcomes at 9-month follow-up. The quadruple composite of cardiac death, MI, definite ST, or TVR occurred in 4.9% with EES versus 5.2% with SES (P=.01 for non-inferiority). TLR rates were 1.4% versus 1.7% (P=.64). In EXCELLENT, the primary outcome was in-segment late lumen loss at 9-month angiographic follow-up. In 935 lesions treated with EES, late lumen loss was 0.10 ± 0.36 mm compared with 0.05 ± 0.34 mm in the 266 SES-treated lesions (P-value for non-inferiority, .023). Furthermore, no differences were observed in clinical outcomes, including TLR (2.4% with EES vs 1.7% with SES; P=.40). In PROENCY, operators preferred SES above EES in cases of long and small lesions, both characteristics associated with a higher risk of restenosis. This might be explained by the unavailability of small or long EES stents at the time of enrollment.
Previous studies. This is one of the few studies comparing EES with SES or ZES. In a recently published study, SES and EES were compared with BMS in large coronary arteries defined as a diameter of > 3 mm.3 TVR rates for reasons unrelated to MI were comparable among patients receiving SES (3.7%) and EES (3.1%). Although trial and registry results may not be directly comparable, and the trial was performed in a population at lower risk of restenosis because of the vessel diameter, the TVR rate in the SES group was lower and the TVR rate in the EES group was higher than those observed in our analysis. Results of LESSON I (Long-term comparison of Everolimus-eluting and Sirolimus-eluting Stents for cOronary revascularisatioN) were recently presented.14 In this propensity-matched analysis of 1342 patient pairs, the use of EES compared with SES was associated with a strong trend toward a lower composite of death, MI, or TVR (14.9% vs 18%; P=.056) and significantly lower MI (3.3% vs 5.0%; P=.017) and TVR rates (7.0% vs 9.6%; P=.039) at 3-year follow-up (median, 1.3 years). Moreover, SES versus EES comparisons have been reported from ISAR TEST IV, Efficacy of Xience/Promus versus Cypher to rEduce Late Loss in stENT (EXCELLENT) and SORT OUT IV.15 In the ISAR TEST IV trial, comparable outcomes were observed at 2-year follow-up. The composite of cardiac death, target vessel MI, or TLR occurred in 16.0% in the EES-treated patients vs 18.8% in the SES-treated patients (P=.23). The TLR rates were 9.9% versus 13.5% (P=.06), respectively. SORT OUT IV showed comparable clinical outcomes at 9-month follow-up. The quadruple composite of cardiac death, MI, definite ST, or TVR occurred in 4.9% with EES versus 5.2% with SES (P=.01 for non-inferiority). TLR rates were 1.4% versus 1.7% (P=.64). In EXCELLENT, the primary outcome was in-segment late lumen loss at 9-month angiographic follow-up. In 935 lesions treated with EES, late lumen loss was 0.10 ± 0.36 mm compared with 0.05 ± 0.34 mm in the 266 SES-treated lesions (P-value for non-inferiority, .023). Furthermore, no differences were observed in clinical outcomes, including TLR (2.4% with EES vs 1.7% with SES; P=.40). In PROENCY, operators preferred SES above EES in cases of long and small lesions, both characteristics associated with a higher risk of restenosis. This might be explained by the unavailability of small or long EES stents at the time of enrollment.
With respect to comparisons of ZES with EES, Serruys et al published the results of the Resolute All Comers multicenter noninferiority trial comparing ZES with EES.12 At 12-month follow-up, no difference was observed in the patient- and lesion-oriented composite outcomes and the individual components, including TVR (4.9% with ZES vs 4.8% with EES) and TLR (3.9% with ZES vs 3.4% with EES). These results are different when compared with the ENDEAVOR III and SORT OUT III trials, indicating a relatively poorer performance of ZES compared with SES regarding TLR at 9-month follow-up.8,10 However, no difference in TLR was observed at 18-month follow-up in the ENDEAVOR III trial, suggesting the absence of the “late-catch” phenomenon with ZES.4,9 A potential explanation for the discrepancy could be the newer biocompatible polymer of the Resolute ZES compared with the Endeavor ZES. The relative poorer performance of the older ZES compared with EES might have led to less ZES placement in patients presenting with a restenotic lesion in our registry.
Altogether, the reduction in the 12-month outcomes in the PROENCY registry is partly achieved by the low repeat revascularization rates with the EES. Although lower than observed in the aforementioned studies, the 2.1% and 1.9% TLR rates are comparable with the 1.9% rate reported in the SPIRIT V single-arm study.16 Recently, a report from the large nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR) showed a lower risk of clinically meaningful restenosis and definite ST when comparing newer-generation DESs (including EES) with older-generation DESs (including the ZES [Endeavor] and SES).17 This corroborates with our current results. Further research is needed to confirm our current data.
Stent thrombosis. Stent thrombosis rates at 1 year were low and comparable between EES and SES or ZES. These results corroborate earlier studies showing comparable ST at 1 year between EES vs ZES12 or EES vs SES.3 However, the use of SES is associated with an increased risk of very late ST. The LESSON I study showed a significantly lower incidence of very late definite ST with EES compared with SES (P=.01).14 In contrast, no difference in ST at 2-year follow-up was observed with SES vs EES in the large coronary artery study.3 At 2-year follow-up in ISAR TEST IV, definite ST rates were 0.6% with EES compared with 1.4% with SES (P=.17). At an absolute level, the EES, ZES, SES definite or probable ST rates are comparable to those reported in an Italian EES registry (0.9%), the ZES E-Five registry (1.1%), and the SES e-Cypher registry (around 0.9%).18-20 Longer follow-up is required for the comparison of very late ST among the “limus” stents in the PROENCY registry.
New stent designs. Since the completion of the PROENCY registry, advances in stent technology have continued in order to improve the clinical outcomes for patients undergoing PCI. In the PLATINUM (a prospective, randomized, multicenter trial to assess an everolimus-eluting coronary stent system [PROMUS Element] for the treatment of up to two de novo coronary artery lesions) trial, a novel platinum chromium EES was non-inferior to the predicate cobalt chromium EES for TLF, with non-significant differences in measures of safety and efficacy through 12-month follow-up after PCI.21 Furthermore, at 12-month follow-up, the novel Resolute ZES was non-inferior to EES in treating “real-world” patients with a vast majority of complex lesions and “off-label” indications for DESs in the TWENTE (randomized controlled trial in second-generation zotarolimus-eluting Resolute stents versus everolimus-eluting Xience V stents in real-world patients) trial.22
Strengths and limitations. Strengths of the PROENCY registry are the prospective design, multicenter, and multinational setting and short patient inclusion period. Several limitations deserve to be mentioned. First, the underreporting of events is an important limitation of all registries. Second, despite propensity-score matching in order to adjust for the non-randomized design of the study, we cannot exclude the possibility of residual confounding. Possible sources of residual bias for the outcomes of cardiac death or MI are the presence of congestive heart failure, infarct size in the patients undergoing urgent PCI for MI, and concomitant therapy. However, regarding restenosis and repeat revascularization, we were able to incorporate important confounders in the propensity score.
Conclusion
In conclusion, the 12-month outcomes of the PROENCY registry show a reduced incidence of the composite of cardiac death, MI, and TVR with EES compared with SES or ZES. This was mainly driven by a reduction in TLR.
References
- Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937-948.
- Kirtane AJ, Gupta A, Iyengar S, et al. Safety and efficacy of drug-eluting and bare-metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119(25):3198-3206.
- Kaiser C, Galatius S, Erne P, et al. Drug-eluting versus bare-metal stents in large coronary arteries. N Engl J Med. 2010;363(24):2310-2319.
- Garg S, Serruys PW. Coronary stents: current status. J Am Coll Cardiol. 2010;56(10 Suppl):S1-S42.
- Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369(9562):667-678.
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356(10):998-1008.
- Mukherjee D, Moliterno DJ. Second-generation drug-eluting stents and the continuous need for rapidly available real-world data. JACC Cardiovasc Interv. 2009;2(12):1236-1239.
- Kandzari DE, Leon MB, Popma JJ, et al. Comparison of zotarolimus-eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trial. J Am Coll Cardiol. 2006;48(12):2440-2447.
- Eisenstein EL, Leon MB, Kandzari DE, et al. Long-term clinical and economic analysis of the Endeavor zotarolimus-eluting stent versus the cypher sirolimus-eluting stent: 3-year results from the ENDEAVOR III trial (Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Cypher Sirolimus-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv. 2009;2(12):1199-1207.
- Rasmussen K, Maeng M, Kaltoft A, et al. Efficacy and safety of zotarolimus-eluting and sirolimus-eluting coronary stents in routine clinical care (SORT OUT III): a randomised controlled superiority trial. Lancet. 2010;375(9720):1090-1099.
- Park DW, Kim YH, Yun SC, et al. Comparison of zotarolimus-eluting stents with sirolimus- and paclitaxel-eluting stents for coronary revascularization: the ZEST (comparison of the efficacy and safety of zotarolimus-eluting stent with sirolimus-eluting and paclitaxel-eluting stent for coronary lesions) randomized trial. J Am Coll Cardiol. 2010;56(15):1187-1195.
- Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med. 2010;363(2):136-146.
- Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344-2351.
- Windecker S. LESSON I (long-term comparison of Everolimus-eluting and Sirolimus-eluting stents for coronary revascularization). Presented at ESC conference 2010 in Stockholm, Sweden.
- Late breaking clinical trials and first report investigations. J Am Coll Cardiol. 2010;56(13 Meeting Abstracts):xii.
- Chevalier B. SPIRIT V single arm study: 2 year follow-up (Abstr). EuroIntervention. 2011.
- Sarno G, Lagerqvist B, Frobert O, et al. Lower risk of stent thrombosis and restenosis with unrestricted use of ‘new-generation’ drug-eluting stents: a report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur Heart J. 2012;33(5):606-613.
- Latib A, Ferri L, Ielasi A, et al. Clinical outcomes after unrestricted implantation of everolimus-eluting stents. JACC Cardiovasc Interv. 2009;2(12):1219-1226.
- Lotan C, Meredith IT, Mauri L, et al. Safety and effectiveness of the endeavor zotarolimus-eluting stent in real-world clinical practice: 12-month data from the E-Five registry. JACC Cardiovasc Interv. 2009;2(12):1227-1235.
- Urban P, Gershlick AH, Guagliumi G, et al. Safety of coronary sirolimus-eluting stents in daily clinical practice: one-year follow-up of the e-Cypher registry. Circulation. 2006;113(11):1434-1441.
- Stone GW, Teirstein PS, Meredith IT, et al. A prospective, randomized evaluation of a novel everolimus-eluting coronary stent: the PLATINUM (a Prospective, Randomized, Multicenter Trial to Assess an Everolimus-Eluting Coronary Stent System [PROMUS Element] for the Treatment of Up to Two de Novo Coronary Artery Lesions) trial. J Am Coll Cardiol. 2011;57(16):1700-1708.
- von BC, Basalus MW, Tandjung K, et al. A randomized controlled trial in second-generation zotarolimus-eluting Resolute stents versus Everolimus-eluting Xience V stents in real-world patients: the TWENTE trial. J Am Coll Cardiol. 2012;59(15):1350-1361.
___________________________________________________________
*Drs Damman, Abdel-Wahab, and Möllmann contributed equally.
From the 1Department of Cardiology, Academic Medical Center – University of Amsterdam, Amsterdam, The Netherlands, 2Heart Center, Segeberger Kliniken GmbH, Academic Teaching Hospital of the University of Kiel, Bad Segeberg, Germany, 3Kerckhoff Klinik, Heart and Thorax Center, Bad Nauheim, Germany, 4Institut Hospitalier Jacques Cartier, Massy, France, 5Polyclinique les Fleurs, Ollioules, France, and 6Boston Scientific Corporation, Natick, Massachusetts.
Funding: Boston Scientific Corporation sponsored the PROENCY Registry.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Abdel-Wahab received honoraria from Boston Scientific and Cordis. Dr Richardt has research contracts with Medtronic and Abbott, and consultant fees from Boston Scientific and Abbott. Dr Barragan received lecture fees from Cordis and Abbott. Dr Hamm received consultant fees from Boston Scientific, Cordis, and Medtronic. Dr Underwood is a full-time employee and stock holder of Boston Scientific. The other authors report no disclosures. Manuscript submitted March 22, 2012, provisional acceptance given May 1, 2012, final version accepted May 16, 2012.
Address for correspondence: Christian Hamm, MD, PhD, FESC, Kerckhoff Heart and Thorax Center, Benekestrasse 2-8, 61231 Bad Nauheim, Germany. Email: c.hamm@Kerckhoff-Klinik.de












