ADVERTISEMENT
Comparison of Angiographic and IVUS Follow-up Between the Two Different Drug-Eluting Stents Implanted Simultaneously in the Same Individuals
Abstract: Background. While, theoretically, a drug-eluting stent (DES) with a biodegradable polymer should reduce the incidence of late in-stent thrombosis, this has not been experimentally tested. Objectives. This study compared long-term manifestations of the Excel DES, with a biodegradable polymer, to the Endeavor DES, with a biocompatible polymer, in the same individuals. Methods. Forty-eight patients underwent simultaneous implantation of 1 or more Endeavor stents and 1 or more Excel stents, during the same procedure, and were evaluated with coronary angiography and intravascular ultrasound (IVUS) at least 1 year postprocedure. Within-patient comparisons were made between the Excel- and Endeavor-stented segments for efficacy and safety. Results. A total of 131 stents (69 Endeavor stents and 62 Excel stents) were implanted in 98 lesions among 48 patients. Baseline characteristics of the lesions in the two stented-segments groups were comparable. Average follow-up duration was 14.3 ± 2.5 months. In-stent late luminal loss and luminal stenosis were higher in Endeavor-stented segments than in Excel-stented segments (P<.01). The binary restenosis rate was slightly higher in Endeavor-stented segments (4.3% vs 1.6%; P=.379). In-stent thrombosis, late incomplete stent apposition, and uncovered stent struts were higher in Excel-stented segments than in Endeavor-stented segments (P<.01). There was 1 case of an in-stent coronary aneurysm with an Excel-stented segment. Four segments, in 4 cases (2 in each stent group), required target lesion revascularization. Conclusion. This study suggested that, compared to DESs with a biocompatible polymer, DESs with biodegradable polymer do not appear to present an advantage for long-term safety.
J INVASIVE CARDIOL 2012;24(8):374-378
Key words: percutaneous coronary intervention, zotarolimus-eluting stent, sirolimus-eluting stent, biodegradable polymer, biocompatible polymer
_______________________________________________________________
The safety and efficacy of drug-eluting stents (DESs) employing a biocompatible polymer, such as the Endeavor stent (Medtronic Vascular), which uses a phosphorylcholine polymer on a cobalt alloy thin-strut stent, have been demonstrated in randomized clinical trials, as well as in real-world practice.1-4 Earlier studies indicated that the durable polymer may play a significant role in DES-related hypersensitivity and delayed vessel healing, which may further contribute to stent thrombosis.5-7 Theoretically, a DES employing a biodegradable polymer should reduce the late in-stent thrombosis rate.8,9 Therefore, biodegradable polymer coatings are now used in the newest generation of DESs.10-12 However, recent angioscopic and optical coherence tomography studies have not supported the advantages of biodegradable polymer over durable polymer.13,14 Therefore, it is necessary to obtain further clinical trial data to test the long-term implications of biodegradable-polymer coated stents.
Currently, randomized, controlled clinical trials and registries are used to evaluate the safety and efficacy of different DESs. However, in these studies, different DESs were compared using grouped patients, who had similar clinical features. Though the baseline differences between groups are counterpoised by statistical methods, the impact of individual variation on the results is unavoidable. Based on these considerations, we set out to investigate the angiographic and intravascular ultrasound (IVUS) manifestations of a DES with a biodegradable polymer (Excel stent; JW Medical System) to a DES with a biocompatible polymer (Endeavor stent) in the same individuals, who were simultaneously implanted with each DES.
Methods
Patient selection. From January 2007 to August 2009, we enrolled patients from Guangzhou General Hospital of Guangzhou Command who had undergone percutaneous coronary intervention (PCI) and who were simultaneously implanted with 1 or more Endeavor stents and 1 or more Excel stents. The stents were not randomly, but optionally, chosen and implanted in same or different vessels. Enrolled patients had to agree to undergo coronary angiographic and IVUS follow-up at least 12 months after the procedure, or sooner if acute coronary symptoms were diagnosed. Patients were excluded from the study if they had more than 2 kinds of stent implanted, if the 2 study stents were implanted during separate interventional procedures, or if they were implanted in a coronary artery bridge vessel or restenotic lesion.
Patients were prescribed at least 12 months of dual antiplatelet therapy, with clopidogrel 75 mg/day and aspirin 100 mg/day. Statins, angiotensin converting enzyme inhibitors, or angiotensin receptor antagonists were used in accordance with accepted guidelines15,16 and the patient’s co-existing diseases and risk stratification.
The Institutional Ethics Committee of Guangzhou General Hospital of Guangzhou Command approved the study protocol. Signed, informed consent was obtained from each patient.
Coronary angiography and IVUS evaluation. Conventional coronary angiography was performed using an AXION Artis dTA digital substraction machine (Siemens). The AXION workstation was employed to undertake angiographic measurements automatically. IVUS examination of the stented vessels was performed after coronary angiography with a 20 MHz rotating transducer (Volcano Avanar 85700; Volcano Corporation) at an automatic pullback velocity of 0.5 mm/s. The images were recorded and then analyzed by two physicians who were blinded to patient information and the stent type used in each lesion. The following parameters were measured from angiographic and IVUS images: in-stent late luminal loss (LLL), luminal stenosis, binary restenosis rate, in-stent thrombosis and aneurysm, mean area stenosis, late incomplete stent apposition, uncovered stent struts, and in-stent parietal thrombus.
In-stent LLL was defined as the difference between the minimal lumen diameter at the stented segment after procedure and at angiographic follow-up. Binary restenosis was defined as ≥50% diameter stenosis at follow-up, and was classified as in-stent if the inside of the stent or the in-segment was located at the stent segment, or up to 5 mm proximal or distal to the stents. Net volume obstruction by volumetric IVUS was defined as the ratio of the neointimal volume to the volume of the stent, multiplied by 100. The measurements at the overlapping segment were ascribed to the two segments of overlapping stents, respectively. An overlapping segment was defined as the overlap of the two stents’ proximal and distal edges, respectively, if the two stents were implanted in series.17Late incomplete stent apposition was defined as 1 or more stent struts clearly separated from the vascular wall, which was not associated with any side branch.18
In-stent thrombosis was diagnosed when one of the two following criteria was met: (1) a visible filling defect was seen within the stent during coronary angiography; or (2) a flash of contrast was seen in the low echo area on IVUS.19
Indications for target lesion revascularization. During the follow-up period, a patient was eligible for target lesion revascularization (TLR) if he/she: (1) had a relapse of angina pectoris or had a positive stress test result and the coronary angiography showed segmental stenosis ≥50% in the target vessels; (2) had an angiogram that showed ≥70% target segment stenosis, but no evidence of chest pain or myocardial ischemia; (3) had an IVUS that revealed ≥75% area stenosis in target segments with or without evidence of chest pain and ischemia; or (4) had an IVUS that revealed serious late incomplete stent apposition or remarkable in-stent thrombus and the interventionist thought re-intervention was necessary. Revascularization of non-target segments was excluded from the TLR analysis.
Statistical analysis. Quantitative data were expressed as mean ± standard deviation and analyzed by student’s t-test. Enumeration data were analyzed by the chi-square and Ridit tests, while u-test was used to compare the rates. Statistical analyses were performed using SPSS 11.0 software (SPSS, Inc). P<.05 was considered to be statistically significant.
Results
Patient demographics. Forty-eight eligible patients were enrolled in this study. The mean age of the cohort was 72 ± 16 years. Over half (39/48) were men. Among the 48 patients, PCI was performed in 12 with acute myocardial infarction, 11 with old myocardial infarction, 16 with unstable angina pectoris, and 9 with stable angina pectoris. Concomitant diseases included hypertension (27 patients), diabetes (21 patients), and hyperlipidemia (31 patients). The mean coronary angiography and IVUS follow-up was 14.3 ± 2.5 months, and the mean duration of dual anti-platelet therapy was 12.8 ± 1.9 months. Three patients discontinued aspirin treatment because of gastrointestinal disturbance at 7, 8, and 10 months after PCI, respectively. One patient underwent coronary angiography ahead of schedule at 9 months due to unstable angina pectoris. The other 44 patients maintained dual anti-platelet therapy with clopidogrel and aspirin for 12 months or longer.
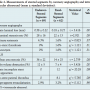
 Lesion characteristics. All 48 patients had multivessel lesions; in total, 98 target lesions were treated with 131 stents (62 Excel stents and 69 Endeavor stents). The target lesions were divided into Endeavor-stented segments and/or Excel-stented segments, based on the type of implanted stent (Table 1). There were no significant differences in the lesion locations or the mean diameters of the target vessels between the two groups (P>.05). The mean stent length of the Endeavor stents was slightly longer than that of the Excel stents (P<.05; Table 2).
Lesion characteristics. All 48 patients had multivessel lesions; in total, 98 target lesions were treated with 131 stents (62 Excel stents and 69 Endeavor stents). The target lesions were divided into Endeavor-stented segments and/or Excel-stented segments, based on the type of implanted stent (Table 1). There were no significant differences in the lesion locations or the mean diameters of the target vessels between the two groups (P>.05). The mean stent length of the Endeavor stents was slightly longer than that of the Excel stents (P<.05; Table 2).
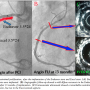
 The angiographic and IVUS measurements are shown in Table 2. LLL, luminal stenosis, mean area stenosis, and net volume obstruction (which indicate neointimal proliferation) were greater in Endeavor-stented segments than in Excel-stented segments (all P<.01). There was a trend, which did not reach statistical significance, toward a higher binary restenosis rate in Endeavor-stented segments than in Excel-stented segments (P>.05). Figure 1 shows a typical presentation of neointimal proliferation in two stented segments. However, the parameters that indicate the safety of DES, such as in-stent thrombosis and late incomplete stent apposition, were higher in Excel-stented segments, compared to Endeavor-stented segments (all P<.01). In-stent thrombosis was detected by IVUS in 5 Excel-stented segments (8.1%) in 5 patients, which was much higher than that detected by coronary angiography (1 segment; 1.6%; P<.05). Four of the 5 patients had no symptoms, while 1 had unstable angina pectoris. Four of the 5 patients were still taking aspirin and clopidogrel when the follow-up coronary angiography was performed. However, clopidogrel was discontinued at 12 months, and a follow-up angiography was performed at 15 months in the remaining patient. An in-stent coronary aneurysm was detected in 1 Excel segment at 14 months after implantation. Four of the 48 patients, 2 in each group, required TLR (8.3%). The reasons for TLR were: restenosis in 2 Endeavor-stented segments and in 1 Excel-stented segment, and 1 in-stent thrombosis in an Excel segment.
The angiographic and IVUS measurements are shown in Table 2. LLL, luminal stenosis, mean area stenosis, and net volume obstruction (which indicate neointimal proliferation) were greater in Endeavor-stented segments than in Excel-stented segments (all P<.01). There was a trend, which did not reach statistical significance, toward a higher binary restenosis rate in Endeavor-stented segments than in Excel-stented segments (P>.05). Figure 1 shows a typical presentation of neointimal proliferation in two stented segments. However, the parameters that indicate the safety of DES, such as in-stent thrombosis and late incomplete stent apposition, were higher in Excel-stented segments, compared to Endeavor-stented segments (all P<.01). In-stent thrombosis was detected by IVUS in 5 Excel-stented segments (8.1%) in 5 patients, which was much higher than that detected by coronary angiography (1 segment; 1.6%; P<.05). Four of the 5 patients had no symptoms, while 1 had unstable angina pectoris. Four of the 5 patients were still taking aspirin and clopidogrel when the follow-up coronary angiography was performed. However, clopidogrel was discontinued at 12 months, and a follow-up angiography was performed at 15 months in the remaining patient. An in-stent coronary aneurysm was detected in 1 Excel segment at 14 months after implantation. Four of the 48 patients, 2 in each group, required TLR (8.3%). The reasons for TLR were: restenosis in 2 Endeavor-stented segments and in 1 Excel-stented segment, and 1 in-stent thrombosis in an Excel segment.
 Overlapping stents were implanted in 21 long lesions, including 8 lesions with 2 Endeavor stents, 6 lesions with 2 Excel stents, and 7 lesions with 1 Endeavor stent and 1 Excel stent. There were no significant differences in late luminal loss, area stenosis rate, or late incomplete stent apposition, between the overlapping segments and non-overlapping segments in the same stent group (all P>.05). One peri-stent aneurysm was observed in the overlapping segment of two Excel stents, which was implanted in the left anterior descending after acute myocardial infarction.
Overlapping stents were implanted in 21 long lesions, including 8 lesions with 2 Endeavor stents, 6 lesions with 2 Excel stents, and 7 lesions with 1 Endeavor stent and 1 Excel stent. There were no significant differences in late luminal loss, area stenosis rate, or late incomplete stent apposition, between the overlapping segments and non-overlapping segments in the same stent group (all P>.05). One peri-stent aneurysm was observed in the overlapping segment of two Excel stents, which was implanted in the left anterior descending after acute myocardial infarction.
Discussion
Biocompatible phosphorylcholine polymer-coated zotarolimus-eluting stents, such as the Endeavor stent, and biodegradable polymer-coated sirolimus-eluting stents, such as the Excel stent, have been widely used in China, as well as in other Asian countries.8,12,20-21 However, there have been no clinical trials directly comparing the safety and efficacy of these two DESs. Furthermore, studies involving long-term angiographic and IVUS follow-up with the Endeavor stent and the Excel stent have been limited to only 6 or 8 months.22-24 In order to directly compare the long-term manifestations of the two DESs, we simultaneously implanted Endeavor and Excel stents during the same index procedure in 48 patients and followed the patients for at least 1 year postprocedure, using coronary angiography and IVUS.
We found that neointimal proliferation was more pronounced in Endeavor-stented segments than in Excel stented-segments. As a result, the LLL, luminal stenosis rate, mean area stenosis rate, and percent neointimal volume obstruction (which indicated neointimal proliferation) were all higher in Endeavor-stented segments. However, most of the segments had only mild stenosis, and only 4.3% of these segments were diagnosed as binary restenosis. The TLR rate was 2.9% in Endeavor-stented segments, which was identical to Excel-stented segments (3.2%). The study also showed that neointimal proliferation resulted in complete coverage of the stent struts and reduced late incomplete stent apposition, which led to a decreased risk of thrombosis. This finding is consistent with the results of the ODESSA trial17,18 and multicenter randomized clinical trials20,25 as well as the “real world” registry data.26
As a DES with a biodegradable polymer, the Excel stent demonstrated a low rate of TLR and thrombosis.12 However, our study also showed that while there was a slightly lower TLR rate with the Excel stent, there was a higher thrombosis rate compared to the Endeavor stent. These findings were probably related to incomplete neointimal proliferation, which increases the rates of late incomplete stent apposition and uncovers stent struts with the Excel stent.
In addition, there was a slightly higher in-stent thrombosis rate, leading to myocardial ischemia with the Excel stent compared to the Endeavor stent. The in-stent thrombosis rate detected by IVUS was much higher with the Excel stent, especially in late stent malapposition segments. Notably, 92% of these patients were taking dual anti-platelet therapy, with clopidogrel and aspirin, when the thrombosis was detected. Thrombotic events could increase after discontinuation of dual anti-platelet therapy during long-term (ie, >12 months) follow-up.27 Theoretically, there should not be any inflammatory reaction after the biodegradable polymer coating is fully absorbed, after 6-9 months,28,29 which might lead to fewer incidences of late thrombosis. This study, however, did not support this proposition and, thus, did not support the superiority of the Excel stent with the biodegradable polymer over the Endeavor stent with the biocompatible polymer. Prospective, randomized, controlled clinical trials are needed to confirm the results of this study.
Study limitations. There were four major limitations of this study. First, this study was not a prospective, randomized, controlled study. The patients were enrolled after the implantation of the studied stents, so the choices of stents were optional, but not random. Second, no baseline IVUS data were available. Third, the drugs of the two studied stents were not identical. Finally, the sample size was small.
Conclusion
The results from this study suggest that greater complete neointimal proliferation increased the potential for restenosis, but not TLR, and might lead to better safety in using Endeavor-stented segments, compared to Excel-stented segments. It may be suggested that, compared to the DES with biocompatible polymer, the DES with biodegradable polymer does not appear to present an advantage for long-term safety.
References
- Leon MB, Mauri L, Popma JJ, et al. A randomized comparison of the ENDEAVOR zotarolimus-eluting stent versus the TAXUS paclitaxel-eluting stent in de novo native coronary lesions 12-month outcomes from the ENDEAVOR IV trial. J Am Coll Cardiol. 2010;55(6):543-554.
- Kandzari DE, Mauri L, Popma JJ, et al. Late-term clinical outcomes with zotarolimus- and sirolimus-eluting stents 5-year follow-up of the ENDEAVOR III (A Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Cypher Sirolimus-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv. 2011;4(5):543-550.
- Fajadet J, Wijns W, Laarman GJ, et al. Long-term follow-up of the randomised controlled trial to evaluate the safety and efficacy of the zotarolimus-eluting driver coronary stent in de novo native coronary artery lesions: five year outcomes in the ENDEAVOR II study. EuroIntervention. 2010;6(5):562-567.
- Jain AK, Lotan C, Meredith IT, et al. Twelve-month outcomes in patients with diabetes implanted with a zotarolimus-eluting stent: results from the E-Five registry. Heart. 2010;9(11):848-853.
- Nebeker JR, Virmani R, Bennett CL, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol. 2006;47(1):175-181.
- Guagliumi G, Farb A, Musumci G, et al. Sirolimus-eluting stent implanted in human coronary artery for 16 months: pathological findings. Circulation. 2003;107(9):1340-1341.
- Fere F, Costa JR, Abizaid A. Very late thrombosis after drug-eluting stents. Catheter Cardiovasc Interv. 2006;68(1):83-88.
- Liu HL, Jin ZG, Luo JP, et al. Long-term effects of biodegradable versus durable polymer-coated sirolimus-eluting stents on coronary arterial wall morphology assessed by virtual histology intravascular ultrasound. Chin Med J. 2011;124(6):836-844.
- Guagliumi G, Sirbu V, Musumeci G, et al. Strut coverage and vessel wall response to a new-generation paclitaxel-eluting stent with an ultrathin biodegradable abluminal polymer: Optical Coherence Tomography Drug-Eluting Stent Investigation (OCTDESI). Circ Cardiovasc Interv. 2010;3(4):367-375.
- Lemos PA, Moulin B, Perin MA, et al. Rationale and design for the PAINT randomized trial. Arq Bras Cardiol. 2009;93:547-553, 590-597.
- Dani S, Kukreja N, Parikh P, et al. Biodegradable-polymer-based, sirolimus-eluting Supralimus stent: 6-month angiographic and 30-month clinical follow-up results from the series I prospective study. EuroIntervention. 2008;4(1):59-63.
- Han Y, Jing Q, Xu B, et al. Safety and efficacy of biodegradable polymer-coated sirolimus-eluting stents in “real-world” practice: 18-month clinical and 9-month angiographic outcomes. JACC Cardiovasc Interv. 2009;2(4):303-309.
- Awata M, Uematsu M, Sera F, et al. Angioscopic assessment of arterial repair following biodegradable polymer-coated biolimus A9-eluting stent implantation. Comparison with durable polymer-coated sirolimus-eluting stent. Circ J. 2011;75(5):1113-1119.
- Guagliumi G, Sirbu V, Musumeci G, et al. Strut coverage and vessel wall response to a new-generation paclitaxel-eluting stent with an ultrathin biodegradable abluminal polymer: Optical Coherence Tomography Drug-Eluting Stent Investigation (OCTDESI). Circ Cardiovasc Interv. 2010;3(4):367-375.
- Hu D, Li J, Li X. China Cholesterol Education Program. Investigation of blood lipid levels and statin interventions in outpatients with coronary heart disease in China: the China Cholesterol Education Program (CCEP). Circ J. 2008;72(6):2040-2045.
- Wang ZW, Wang X, Zhang LF, et al. Hypertension Control in Communities (HCC): evaluation result of blood pressure management among hypertensive. Zhonghua Liu Xing Bing Xue Za Zhi. 2010;31(1):1-4.
- Tahara S, Bezerra HG, Sirbu V, et al. Angiographic, IVUS, and OCT evaluation of the long-term impact of coronary disease severity at the site of overlapping drug-eluting and bare metal stents: a substudy of the ODESSA trial. Heart. 2010;96(19):1574-1578.
- Guagliumi G, Musumeci G, Sirbu V, et al. Optical coherence tomography assessment of in vivo vascular response after implantation of overlapping bare-metal and drug-eluting stents. JACC Cardiovasc Interv. 2010;3(5):531-539.
- Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies. J Am Coll Cardiol. 2001;37(5):1478-1492.
- Xu B, Dou KF, Han YL, et al. A prospective multicenter parallel-controlled trial of TIVOLI biodegradable-polymer-based sirolimus-eluting stent compared to ENDEAVOR zotarolimus-eluting stent for the treatment of coronary artery disease: 8-month angiographic and 2-year clinical follow-up results. Chin Med J (Engl). 2011;124(6):811-816.
- Tian F, Chen YD, Chen L, et al. Evaluation on the neointimal coverage post drug-eluting stent implantation by optical coherence tomography. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39(3):204-207.
- Park SM, Kim JS, Ko YG, et al. Angiographic and intravascular ultrasound follow-up of paclitaxel- and sirolimus-eluting stent after poststent high-pressure balloon dilation: from the poststent optimal stent expansion trial. Catheter Cardiovasc Interv. 20111;77(1):15-21.
- Doi H, Maehara A, Mintz GS, et al. Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents: an integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. JACC Cardiovasc Interv. 2009;2(12):1269-1275.
- Verheye S, Agostoni P, Dubois CL, et al. 9-month clinical, angiographic, and intravascular ultrasound results of a prospective evaluation of the Axxess self-expanding biolimus A9-eluting stent in coronary bifurcation lesions: the DIVERGE (Drug-Eluting Stent Intervention for Treating Side Branches Effectively) study. J Am Coll Cardiol. 2009;53(12):1031-1039.
- Fajadet J, Wijns W, Laarman GJ, et al. Randomized, double-blind, multicenter study of the ENDEAVOR zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions. Circulation. 2006;114(8):798-806.
- Hahn JY, Song YB, Choi JH, et al. Three-month dual antiplatelet therapy after implantation of zotarolimus-eluting stents: the DATE (Duration of Dual Antiplatelet Therapy After Implantation of Endeavor Stent) registry. Circ J. 2010;74(11):2314-2321.
- Grines CL, Bonow RO, Casey DE Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Circulation. 2007;115(6):813-818.
- Parker T, Davé V, Falotico R. Polymers for drug eluting stents. Curr Pharm Des. 2010;16(36):3978-3988.
- Xi TF, Gao RL, Xu B, et al. In vitro and in vivo changes to PLGS/sirolimus coating on drug eluting stents. Biomaterials 2010; 31: 5151-8.
_______________________________________________________________
From the Liuhuaqiao Hospital, Department of Cardiology, Guangzhou, Guangdong, China.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Xiang is a member of the Medtronic Vascular Speaker’s Bureau and has received travel expenses from Medtronic Vascular and Jiwei Medical. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted November 29, 2011, provisional acceptance given January 17, 2012, final version accepted April 9, 2012.
Address for correspondence: Prof. Dingcheng Xiang, MD, PhD, Liuhuaqiao Hospital, Department of Cardiology, #111, Liuhua Rd, Guangzhou, Guangdong 510010, China. Email: dcxiang@foxmail.com












