Clinical Safety of Bivalirudin in Patients Undergoing Carotid Stenting
Abstract: Background. Prior to June 2011, carotid artery stenting (CAS) had been limited to patients deemed high risk for surgical revascularization due to medical or anatomic reasons. Intraprocedural anticoagulation for CAS has traditionally been carried out with unfractionated heparin (UFH). The direct thrombin inhibitor bivalirudin has emerged as a possible alternative choice for anticoagulation in this patient population. In patients undergoing coronary interventions, bivalirudin has been shown in large prospective analysis to reduce major adverse events and hemorrhagic complications (TIMI major bleeding rates, 0.6%-3.1%; TIMI minor bleeding rates, 1.3%-3.7%). As of now, the safety and efficacy of bivalirudin for use during carotid stenting has not been rigorously evaluated. To date, the published evidence in favor of bivalirudin for CAS exists in small retrospective analyses and two prospective studies. Methods. We present a retrospective analysis of 331 patients with a total of 365 carotid artery lesions undergoing CAS between February 2007 and September 2010. The procedures were performed by five experienced operators from four separate sites within the same metropolitan area. Patients were included who received bivalirudin as the anticoagulation strategy and underwent CAS. The primary endpoints of the study were 30-day incidence of death, stroke, TIMI major bleeding (defined as ≥5 g/dL Hgb drop or intracranial hemorrhage), TIMI minor bleeding (defined as ≥3 g/dL Hgb drop), and blood transfusion. All data were collected by retrospective chart review. Results. A total of 365 CAS procedures were performed. There were no deaths, strokes, or TIMI major bleeds. There was a 2.19% incidence of TIMI minor bleeding (8/365) and a 1.64% rate of blood transfusion (6/365). Conclusions. In our patient population, the major endpoints of stroke, death, MI, major and minor bleeding rates were well within those previously reported overall for carotid artery revascularization. Hence, we conclude that bivalirudin may be safe for use in CAS procedures with a safety profile similar to that validated in percutaneous coronary interventions.
J INVASIVE CARDIOL 2012;24(5):202-205
Key words: bivalirudin, thrombin antagonist, carotid artery stenting
_________________________________________
Carotid artery stenting (CAS) is an alternative to carotid endarterectomy (CEA) for patients with severe carotid artery stenosis. Prior to June 2011, CAS was reserved for patients thought to be at high risk for CEA due to medical and/or anatomic reasons. Traditionally, unfractionated heparin (UFH) has served as the anticoagulant of choice. UFH directly activates platelets, promotes platelet aggregation, and thereby may aid in propagation of thrombus. UFH also binds to plasma proteins, resulting in a variable anticoagulant response. By comparison, the direct thrombin inhibitor (DTI), bivalirudin, has weak antiplatelet actions and does not bind to plasma proteins, which confers a more predictable dose-response profile and linear pharmacokinetics.1-3 Bivalirudin inhibits both circulating and fibrin-bound thrombin, and in randomized trials of patients undergoing percutaneous coronary intervention (PCI) for both stable and acute coronary syndromes has resulted in a lower incidence of bleeding with a similar incidence of ischemic complications.4-7
While the safety of bivalirudin has been extensively studied in PCI, limited data are available comparing bivalirudin to UFH in CAS.8-10 There have been only two prospective studies8,9 on the safety of DTIs for CAS. The aim of this study was to explore the safety and efficacy of bivalirudin in CAS patients. We examined periprocedural and 30-day clinical outcomes of patients undergoing CAS using bivalirudin.
Methods
 Patient population. This study is a retrospective analysis of 331 patients with a total of 365 lesions undergoing CAS procedures performed between February 2007 and September 2010. Five experienced operators from four separate sites performed all of the CAS procedures. Sites included two academic hospitals and two private institutions within the same metropolitan area. Patients were candidates for revascularization if noninvasive imaging revealed >50% carotid lumen diameter stenosis in symptomatic patients, or >70% stenosis of one or both carotid arteries in asymptomatic patients. All patients had subselective cerebro-cervical angiography to define vessel anatomy and determine anatomic suitability for percutaneous intervention. Patients were deemed unsuitable for CAS if lesion severity did not meet prescribed angiographic criteria, or if aortic arch or carotid artery anatomy was excessively tortuous and/or heavily calcific. Consecutive patients who received bivalirudin as the anticoagulation strategy formed the study sample.
Patient population. This study is a retrospective analysis of 331 patients with a total of 365 lesions undergoing CAS procedures performed between February 2007 and September 2010. Five experienced operators from four separate sites performed all of the CAS procedures. Sites included two academic hospitals and two private institutions within the same metropolitan area. Patients were candidates for revascularization if noninvasive imaging revealed >50% carotid lumen diameter stenosis in symptomatic patients, or >70% stenosis of one or both carotid arteries in asymptomatic patients. All patients had subselective cerebro-cervical angiography to define vessel anatomy and determine anatomic suitability for percutaneous intervention. Patients were deemed unsuitable for CAS if lesion severity did not meet prescribed angiographic criteria, or if aortic arch or carotid artery anatomy was excessively tortuous and/or heavily calcific. Consecutive patients who received bivalirudin as the anticoagulation strategy formed the study sample.
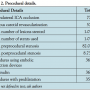 Data collection. Data pertaining to patient demographics (including age, gender, tobacco use, and co-morbid conditions), clinical characteristics, and procedural data were collected by retrospective review of patient charts (Table 1). Clinical data included preprocedure and 24-hour postprocedure hemoglobin and blood indices, type and dose of antithrombin agent. Patient hospital records were audited to document cerebrovascular complications, myocardial infarction (MI), and incidence of bleeding events requiring therapy before discharge. All patients were clinically followed for predefined outcome measures at 30 days postprocedure. The study was approved by each individual hospital’s institutional review board (Tables 2 and 3).
Data collection. Data pertaining to patient demographics (including age, gender, tobacco use, and co-morbid conditions), clinical characteristics, and procedural data were collected by retrospective review of patient charts (Table 1). Clinical data included preprocedure and 24-hour postprocedure hemoglobin and blood indices, type and dose of antithrombin agent. Patient hospital records were audited to document cerebrovascular complications, myocardial infarction (MI), and incidence of bleeding events requiring therapy before discharge. All patients were clinically followed for predefined outcome measures at 30 days postprocedure. The study was approved by each individual hospital’s institutional review board (Tables 2 and 3).
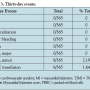 Definitions. Major stroke was defined as acute neurological deficit and altered NIH stroke score by >4 with symptoms persisting at 30 days. Minor stroke was defined as new neurological deficit that either resolved completely within 30 days or increased the NIH stroke scale by <3. Transient ischemic attack was defined as focal retinal or hemispheric event from which the patient made complete recovery within 24 hours.8Carotid artery stenosis before and after stenting was defined according to the NASCET Criteria.11TIMI major bleeding was defined as ≥5 g/dL Hgb drop or intracranial hemorrhage. TIMI minor bleeding was defined as ≥3 g/dL Hgb drop.
Definitions. Major stroke was defined as acute neurological deficit and altered NIH stroke score by >4 with symptoms persisting at 30 days. Minor stroke was defined as new neurological deficit that either resolved completely within 30 days or increased the NIH stroke scale by <3. Transient ischemic attack was defined as focal retinal or hemispheric event from which the patient made complete recovery within 24 hours.8Carotid artery stenosis before and after stenting was defined according to the NASCET Criteria.11TIMI major bleeding was defined as ≥5 g/dL Hgb drop or intracranial hemorrhage. TIMI minor bleeding was defined as ≥3 g/dL Hgb drop.
Endpoints. The primary endpoints of the study were: (1) 30 day incidence of death, stroke, and myocardial infarction; (2) TIMI major bleeding (defined as ≥5 g/dL Hgb drop or intracranial hemorrhage); (3) TIMI minor bleeding (defined as ≥3 g/dL Hgb drop); and (4) blood transfusion.
Carotid stenting protocol. All patients were referred after clinical evaluation and noninvasive imaging revealed significant carotid stenosis. Patients were premedicated with aspirin 325 mg and clopidogrel loading dose (600 mg or 300 mg) before the intervention. NIH stroke scale was determined immediately before the procedure and within 24 hours following. Patients were not sedated for the procedure. Neurological assessment was performed at frequent intervals during the procedure. Hemodynamics and oxygen saturation were continuously monitored. Cervico-cerebral angiography was performed according to standard techniques, using conventional views to define aberrant vascular supply.
Procedural technique. Bivalirudin (0.75 mg/kg bolus, followed by a maintenance infusion of 1.75 mg/kg) was administered immediately after diagnostic angiography was performed. The bivalirudin infusion was discontinued at the end of the procedure. A distal embolic protection device was used in all carotid interventions. Typically, pre- and post-dilatations were performed using 3.0-3.5 and 5.0-5.5 mm balloons, respectively, and with single balloon inflations only. At the conclusion of the carotid procedure, common femoral artery angiography was performed to confirm suitability for arteriotomy closure using a vascular closure device (VCD). It was deemed suitable if the site of sheath insertion into the common femoral artery was above the bifurcation of the superficial femoral and profunda arteries. If appropriate, vascular closure was performed using a Perclose (Abbott Vascular Inc) or AngioSeal VCD (St. Jude Medical Inc). Patients recovered in a monitored environment with experienced nursing and medical staff. Ambulation was encouraged 1 hour post vascular closure if VCD was used. Patients were routinely discharged the next day and were prescribed combination aspirin and clopidogrel for at least 4 weeks, and then aspirin indefinitely.
Statistical analysis. Statistical analysis was performed using Excel (Microsoft Corporation). Statistical analyses included calculation of mean, percentage, and other basic arithmetic values. The authors had full access to the data and take responsibility for its accuracy and integrity.
Results
There were a total of 365 procedures performed (Table 1) on 331 patients between February 2007 and September 2010. Mean age of the patients was 70.3 years, and 268 patients (73.4%) were male. Restenosis after previous carotid revascularization was treated in 32 patients (15.5%), and contralateral occlusion was found in 49 patients (23.7%).
Procedural success. CAS was successfully completed in all 365 procedures (100%). Mean number of stents used was 1.074 and mean number of lesions stented was 1.074 (Table 2). The mean stenosis before stenting was 82.07%, and mean stenosis after stenting was 8.73%. Predilatation was performed in 358 procedures. All procedures used bivalirudin as the anticoagulant. Distal embolic protection devices were used in all 365 procedures.
Procedural and in-hospital outcomes. There were no periprocedural deaths, major strokes, or minor strokes. In addition, there were no intraprocedural transient ischemic events. None of the patients experienced intraprocedural or postprocedural myocardial infarction. There were no major bleeding complications. Also, there were no hospital readmissions (over a 30-day period) postprocedure. There were 8 episodes of TIMI minor bleeding complications (2.19%) documented within the first 24 hours. Six of these required blood transfusion (1.64%).
Thirty-day follow-up (Table 3). Thirty-day follow-up was obtained in each patient (n = 331) following all 365 carotid stent procedures. There were no neurological events, MIs, or deaths during that time. Additionally, there were no further episodes of TIMI major bleeding, TIMI minor bleeding, or need for blood transfusion.
Discussion
CAS is an alternative treatment to carotid endarterectomy for symptomatic carotid stenosis in high-risk patients with favorable anatomy, as demonstrated by a few randomized controlled trials. CAS has benefitted over time from better patient selection, widespread use of embolic protection, and increased levels of operator experience. In fact, studies published in the last several years have demonstrated that CAS is a safe procedure with acceptable rates of embolic phenomena and low rates of neurological sequelae.8-16
The stents used in carotid interventions have bare-metal surface area that is significantly more than coronary stents and hence have the added potential for thrombogenicity. Several studies using diffusion-weighted magnetic resonance imaging have shown a high incidence of micro-emboli during CAS.17 Although rare, there have been a few case reports of acute carotid stent thrombosis.18 In this scenario, a DTI, such as bivalirudin, in conjunction with dual antiplatelet blockade with aspirin and clopidogrel, may prove to be efficacious in preventing thrombus formation during CAS.
DTIs inhibit clot-bound thrombin more effectively than UFH and are not inactivated by plasma proteins or platelet factor 4. Bivalirudin is a synthetic derivative of hirudin, with a shorter half life, that reversibly inhibits thrombin. In the ACUITY trial, which studied patients with unstable angina/non-ST elevation myocardial infarction, the clinical efficacy of bilavirudin plus glycoprotein (GP) IIb/IIIa was comparable to the combination of UFH and GP IIb/IIIa with regard to 30-day history of ischemia (7.7% vs 7.3%, respectively). Also, in patients who received thienopyridines before PCI, bivalirudin alone has shown to have comparable efficacy to the combination of UFH and GP IIb/IIIa with regard to the combined ischemic and hemorrhagic endpoints of death, myocardial infarction, unplanned urgent revascularization, and major bleeding at the time of PCI, with lower bleeding complications as compared to the latter.6
Initial studies on the use of bivalirudin in CAS date back to 2005 when Lin et al performed a prospective study19 aimed at assessing the effect of the learning curve on treatment complications and the clinical outcomes of CAS. As a part of this study, four groups (each containing 50 patients) underwent CAS with the first 54 consecutive patients receiving UFH. In light of the success of bivalirudin in the REPLACE-2 trial, the rest of the 146 patients received bivalirudin as the anticoagulant during the periprocedural period. This decreased bleeding complications in groups III and IV (P=.03) compared with group I. The modification of the anticoagulation resulted in a significant decrease in hemorrhagic complications (6% of group I patients [3 of 50] and 2% of group II patients [1 of 50] and 0 complications in the latter 2 patient groups).The 30-day stroke and death rate in groups I and II was 8% and 2%, respectively, and was decreased significantly in groups III and IV (0% and 0%, respectively; P<.05).19
Cogar et al presented a retrospective analysis of 206 patients who underwent CAS procedures using bivalirudin between February 2007 and September 2009. There were no deaths, strokes, or TIMI major bleeding events. There was a 3.5% incidence of TIMI minor bleeding (8/230) and a 2.6% rate of blood transfusion (6/230).10 Folmar et al published data on 42 patients who were selected to undergo CAS via transradial approach from January 2005 to August 2006 with bivalirudin as the anticoagulation of choice. All patients had a carotid artery stenosis of >80% by arteriography and comorbid conditions increasing the risk of carotid endarterectomy. There were no radial access-site complications. One patient sustained a stroke 24 hours after the procedure with complete resolution of symptoms (mean NIH stroke scale, 2.0 ± 0.3 before and 1.9 ± 0.3 after).20
Schneider et al recently reported data pertaining to 3-year analysis of procedural outcomes regarding use of bivalirudin in 512 patients with or without vascular closure devices. Thirty-day stroke and death rate was 1.7%. There were 4 major bleeding complications requiring transfusion (0.7%), and length of stay was delayed more than 24 hours in 5 patients (0.93%), all of whom were in the manual compression group.8 Stabile et al randomized 220 patients to bivalirudin vs UFH during CAS with proximal embolic protection and assessed their relative safety and efficacy. There was no difference in 30-day mortality or cerebrovascular outcomes. The bivalirudin cohort had significantly fewer procedure-related TIMI major and minor bleeding complications (7.3 vs 16.4%; P<.05).9
Study limitations. Given that the data we present are from a retrospective observational analysis, the major limitation of this study would be the lack of randomization and control group. In this scenario, it would be difficult to delineate superiority of one anticoagulation regimen over the other. Our results do, however, indicate a trend toward better outcomes with regard to stroke, death, and procedural bleeding with CAS performed at our centers by our five operators as compared to outcomes of CEA/CAS with heparin published in medical literature.
Conclusion
Use of bivalirudin as an anticoagulant for CAS proved to be safe and effective in our study. Compared to statistics accrued from previous studies, bivalirudin may prove to have a better outcome profile compared to CEA/CAS with UFH. However, we need further randomized control trials to bolster these findings and to provide further insight into the efficacy of bivalirudin in CAS procedures.
In our experience, bivalirudin is an effective intraprocedural anticoagulation strategy when performing CAS. In our study, the major endpoints like stroke, death, MI, and access-site bleeding rates were well within acceptable guidelines reported for carotid artery revascularization. Hence, we conclude that bivalirudin may be safe for use in carotid artery stenting procedures with a safety profile similar to that validated in percutaneous coronary interventions.
References
- Anand SX, Kim MC, Kamran M, et al. Comparison of platelet function and morphology in patients undergoing percutaneous coronary intervention receiving bivalirudin versus unfractionated heparin versus clopidogrel pretreatment and bivalirudin. Am J Cardiol. 2007;100(3):417-424.
- Lepor NE. Anticoagulation for acute coronary syndromes: from heparin to direct thrombin inhibitors. Rev Cardiovasc Med. 2007;8(Suppl 3):S9-S17.
- Hirsh J, O’Donnell M, Eikelboom J. Beyond unfractionated heparin and warfarin: current and future advances. Circulation. 2007;116(5):552-560.
- Gibson CM, Ten Y, Murphy SA, et al. Association of prerandomization anticoagulant switching with bleeding in the setting of percutaneous coronary intervention (a REPLACE-2 analysis). Am J Cardiol. 2007;99(12):1687-1690.
- Schulz S, Mehilli J, Ndrepepa G, et al. Intracoronary stenting and antithrombotic regimen: rapid early action for coronary treatment (ISAR-REACT) 3 trial investigators. Bivalirudin vs. unfractionated heparin during percutaneous coronary interventions in patients with stable and unstable angina pectoris: 1-year results of the ISAR-REACT 3 trial. Eur Heart J. 2010;31(5):582-587.
- Lopes RD, Alexander KP, Manoukian SV, et al. Advanced age, antithrombotic strategy, and bleeding in non-ST segment elevation acute coronary syndromes: results from the ACUITY (acute catheterization and urgent intervention triage strategy) trial. J Am Coll Cardiol. 2009;53(12):1021-1030.
- Stone GW, Witzenbichler B, Guagliumi G, et al; the HORIZONS-AMI trial investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358(21):2218-2230.
- Schneider LM, Polena S, Roubin G, et al. Carotid stenting and bivalirudin with and without vascular closure: 3-year analysis of procedural outcomes. Catheter Cardiovasc Interv. 2010;75(3):420-426.
- Stabile E, Sorropago G, Tesorio T, et al. Heparin versus bivalirudin for carotid artery stenting using proximal endovascular clamping for neuroprotection: results from a prospective randomized study. J Vasc Surg. 2010;52(6):1505-1510.
- Cogar BD, Abu-Fadel M, Hennebry TA, Chrysant GS. Clinical safety of bivalirudin in patients undergoing carotid stenting. Poster presentation at the SCAI 33rd Annual Scientific Sessions, San Diego, CA: May 5-8, 2010.
- Moneta GL, Edwards JM, Chitwood RW, et al. Correlation of North American symptomatic carotid endarterectomy trial (NASCET) angiographic definition of 70% to 99% internal carotid artery stenosis with duplex scanning. J Vasc Surg. 1993;17(1):152-157; discussion, 157-159.
- Roubin GS, New G, Iyer SS, et al. Immediate and late clinical outcomes of carotid artery stenting in patients with symptomatic and asymptomatic carotid artery Stenosis: a 5-year prospective analysis. Circulation. 2001;103(4):532-537.
- Yadav JS, Wholey MH, Kuntz RE, et al. Stenting and angioplasty with protection in patients at high risk for endarterectomy investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351(15):1493-1501.
- Ahrens I, Smith BK, Bode C, Peter K. Direct thrombin inhibition with bivalirudin as an antithrombotic strategy in general and interventional cardiology. Expert Opin Drug Metab Toxicol. 2007;3(4):609-620.
- Shammas NW. Bivalirudin: pharmacology and clinical applications. Cardiovasc Drug Rev. 2005;23(4):345-360.
- Brott TG, Hobson RW, Howard G, et al. Stenting versus endarterectomy for treatment of carotid artery stenosis. N Engl J Med. 2010;363(1):11-23.
- Tedesco MM, Lee JT. Postprocedural microembolic events following carotid surgery and carotid angioplasty and stenting. J Vasc Surg. 2007;46(2):244-250.
- Setacci C, De Donato G, Setacci F. Surgical management of acute carotid thrombosis after carotid stenting: a report of three cases. J Vasc Surg. 2005;42(5):993-996.
- Lin PH, Bush RL, Peden EK, et al. Carotid artery stenting with neuroprotection: assessing the learning curve and treatment outcome. Am J Surg. 2005;190(6):850-857.
- Folmar J, Sachar R, Mann T. Transradial approach for carotid artery stenting: a feasibility study. Catheter Cardiovasc Interv. 2007;69(3):355-361.
_________________________________________
From the 1Integris Baptist Medical Center, 2Oklahoma Heart Hospital, and 3University of Oklahoma Health Sciences Center, Oklahoma City VA Medical Center, Oklahoma City, Oklahoma.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein. Dr Chrysant is a consultant for St Jude and Abbott Vascular and is on the Speaker’s Bureau for Abbott Vascular. Dr Hennebry is a proctor for Abbott Vascular.
Manuscript submitted November 29, 2011, provisional acceptance given January 2, 2012, final version accepted February 7, 2012.
Address for correspondence: George S. Chrysant, MD, Director, Peripheral Interventions and Advanced Cardiac Imaging, INTEGRIS Baptist Medical Center, Clinical Associate Professor of Medicine, University of Oklahoma, 3433 NW 56th Street, Building B, Suite 660, Oklahoma City, OK 73112. Email: gsc5@yahoo.com












