ADVERTISEMENT
Bilateral Arm Approach for Percutaneous Coronary Intervention of Unprotected Left Main Supported by a Modified Intra-Aortic Balloon Pump
Abstract: Patients in whom femoral arterial access is not feasible pose a challenge in terms of hemodynamic support during high-risk percutaneous coronary intervention. Patient’s height adds another challenge given the fixed lengths of available intra-aortic balloon pumps, in terms of achieving an adequate infrasubclavian positioning in the descending thoracic aorta. We report a case where a modified intra-aortic balloon pump helped achieve a successful result in a patient undergoing intervention of an unprotected left main using bilateral arm approach.
J INVASIVE CARDIOL 2012;24(4):183-184
Key words: unprotected left main, intra-aortic balloon pump, brachial approach, percutaneous coronary intervention
____________________________________________
Prophylactic transbrachial placement of intra-aortic balloon pump (IABP) for high risk coronary artery bypass grafting is well known. We hereby report our experience of transbrachial placement of a modified intra-aortic balloon pump in a patient during high-risk percutaneous coronary intervention of unprotected left main coronary artery.
Case Report
 A 72-year-old Caucasian with coronary artery bypass grafting (CABG) 20 years ago, morbid obesity, occluded infrarenal abdominal aorta (history of aorto-bifemoral bypass grafting), and diabetes presented with non-ST elevation myocardial infarction (MI) and new-onset congestive heart failure. Physical examination revealed bilateral rales and 2+ pitting edema of lower extremities. Femoral pulses were barely palpable while other lower extremity pulses were not palpable. He was 72 inches tall, weight was 383 lb, and body mass index was 45.
A 72-year-old Caucasian with coronary artery bypass grafting (CABG) 20 years ago, morbid obesity, occluded infrarenal abdominal aorta (history of aorto-bifemoral bypass grafting), and diabetes presented with non-ST elevation myocardial infarction (MI) and new-onset congestive heart failure. Physical examination revealed bilateral rales and 2+ pitting edema of lower extremities. Femoral pulses were barely palpable while other lower extremity pulses were not palpable. He was 72 inches tall, weight was 383 lb, and body mass index was 45.
Electrocardiogram showed sinus rhythm, right bundle branch block and 1 mm ST elevation in lead aVR (new since baseline). Lab studies showed creatinine of 2.0 mg/dL, elevated Troponin I (7.6 ng/mL), creatine kinase 307, and creatine kinase-myocardial band was 58. Left ventricular ejection fraction was 30% by echocardiography.
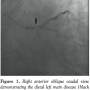
 Attempt to obtain arterial access via femoral route through pre-existing aorto-femoral bypass graft bilaterally was unsuccessful. Using a left brachial approach, coronary angiography revealed severe distal left main stenosis (75%) extending into the proximal left anterior descending (LAD) artery (Figure 1), a patent large intermediate ramus was patent; and chronic total occlusion of a large obtuse marginal branch and right coronary artery. All bypass grafts were occluded. His balloon-pump assisted coronary intervention1 jeopardy score (BCIS-1) was 12 (maximum). Left ventricular end-diastolic pressure was 28 mm Hg. Abdominal aortography showed total occlusion of infrarenal aorta with total occlusion of aorto-bifemoral grafts (see Video 1 at www.invasivecardiology.com).Cardiothoracic surgery was consulted, but due to comorbidities, the patient was not deemed a candidate for repeat CABG. An upper extremity arterial duplex scan performed to ascertain that the left brachial artery was large enough to accommodate the IABP revealed a size of 5.8 x 6.2 mm.
Attempt to obtain arterial access via femoral route through pre-existing aorto-femoral bypass graft bilaterally was unsuccessful. Using a left brachial approach, coronary angiography revealed severe distal left main stenosis (75%) extending into the proximal left anterior descending (LAD) artery (Figure 1), a patent large intermediate ramus was patent; and chronic total occlusion of a large obtuse marginal branch and right coronary artery. All bypass grafts were occluded. His balloon-pump assisted coronary intervention1 jeopardy score (BCIS-1) was 12 (maximum). Left ventricular end-diastolic pressure was 28 mm Hg. Abdominal aortography showed total occlusion of infrarenal aorta with total occlusion of aorto-bifemoral grafts (see Video 1 at www.invasivecardiology.com).Cardiothoracic surgery was consulted, but due to comorbidities, the patient was not deemed a candidate for repeat CABG. An upper extremity arterial duplex scan performed to ascertain that the left brachial artery was large enough to accommodate the IABP revealed a size of 5.8 x 6.2 mm.
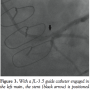 A 7 Fr sheath was placed in the left brachial artery. Initially, a 40 cc IABP was placed through the brachial sheath. Since the patient was a taller person, it was difficult to achieve proper placement distal to the origin of the left subclavian artery due to the length of the IABP even when it was “hubbed,” and even after changing over to a 30 cc IABP (Datascope, Maquet Corp) and using a sheathless insertion, one end of the IABP remained within the left subclavian artery. To achieve accurate placement, the 5 cm protective covering at the proximal edge
A 7 Fr sheath was placed in the left brachial artery. Initially, a 40 cc IABP was placed through the brachial sheath. Since the patient was a taller person, it was difficult to achieve proper placement distal to the origin of the left subclavian artery due to the length of the IABP even when it was “hubbed,” and even after changing over to a 30 cc IABP (Datascope, Maquet Corp) and using a sheathless insertion, one end of the IABP remained within the left subclavian artery. To achieve accurate placement, the 5 cm protective covering at the proximal edge  of the balloon pump was peeled off and removed, which allowed adequate placement into the descending aorta distal to the left subclavian artery (Figure 2; see Video 2 at www.invasivecardiology.com). Excellent diastolic augmentation was achieved and the arterial waveform of diastolic augmentation was identical to the usual waveform, despite the fact that the IABP was “upside-down.” Pulse oximetry on the left middle finger was used constantly to ascertain normal arterial perfusion. A 6 Fr sheath was placed in the right radial artery. After trying multiple guide catheters and failing to selectively engage the ostium of the left main, we finally were able to get close enough to the left main ostium with a JL-3.5 guide catheter and using an Asahi Prowater (Abbott Vascular), we utilized the “fetching with the wire” technique to fetch the left main and were able to advance the guidewire into the distal LAD. Then, we were able to selectively engage the left main using the wire as a support. A 4.0 x 15 mm Xience stent (Abbott Vascular) was deployed in the distal left main extending into the LAD (Figure 3). There was a distal stent edge dissection of the proximal LAD, and therefore, another 3.5 x 12 mm Xience stent was deployed in the proximal LAD in an overlapping fashion. Final angiography revealed TIMI-3 flow, in LAD, preservation of flow into the large intermediate ramus, and no residual stenosis or dissection (Figure 4; see Video 3 at www.invasivecardiology.com). IABP was removed in the laboratory. The patient is doing well 8 months after the procedure with no repeat hospitalization or revascularization.
of the balloon pump was peeled off and removed, which allowed adequate placement into the descending aorta distal to the left subclavian artery (Figure 2; see Video 2 at www.invasivecardiology.com). Excellent diastolic augmentation was achieved and the arterial waveform of diastolic augmentation was identical to the usual waveform, despite the fact that the IABP was “upside-down.” Pulse oximetry on the left middle finger was used constantly to ascertain normal arterial perfusion. A 6 Fr sheath was placed in the right radial artery. After trying multiple guide catheters and failing to selectively engage the ostium of the left main, we finally were able to get close enough to the left main ostium with a JL-3.5 guide catheter and using an Asahi Prowater (Abbott Vascular), we utilized the “fetching with the wire” technique to fetch the left main and were able to advance the guidewire into the distal LAD. Then, we were able to selectively engage the left main using the wire as a support. A 4.0 x 15 mm Xience stent (Abbott Vascular) was deployed in the distal left main extending into the LAD (Figure 3). There was a distal stent edge dissection of the proximal LAD, and therefore, another 3.5 x 12 mm Xience stent was deployed in the proximal LAD in an overlapping fashion. Final angiography revealed TIMI-3 flow, in LAD, preservation of flow into the large intermediate ramus, and no residual stenosis or dissection (Figure 4; see Video 3 at www.invasivecardiology.com). IABP was removed in the laboratory. The patient is doing well 8 months after the procedure with no repeat hospitalization or revascularization.
Discussion
Hemodynamic support of patients undergoing high-risk PCI is the most common reason to place an IABP.2 These patients are perceived to be at high risk of developing complications during PCI.
Placement of IABP through the native common femoral artery is by far the most common approach. Presence of advanced peripheral arterial disease renders this approach improbable. One reported alternative to this approach is surgical insertion of IABP into the axillary artery,3 which involves a surgical cut-down and therefore, logistically is not the most feasible in the current era. Prosthetic aorto-femoral bypass grafts have been utilized for placement of IABP without major complications.4 Two previous cases utilized brachial approach for IABP support of high-risk PCI using a left brachial approach.5,6 Although the height of the patient was not reported, they did not report any issues with placement of a 40 cc IABP using the brachial approach. However, our patient was different in the sense that height of the patient did not allow placement of a 40 cc IABP, which would have been appropriate for his height. Therefore, sheathless use of a 30 cc IABP and then, removal of the proximal covering allowed appropriate placement with satisfactory diastolic augmentation. To our knowledge, this is the first report of a modified IABP used via a brachial approach. Ascertaining the size of brachial artery to assess feasibility of using a larger sheath size, as needed for IABP, appears to be a safer approach. Additionally, the arterial duplex scan of the upper extremity would help exclude any significant subclavian artery stenosis, which is present in approximately 6% of patients with coronary artery disease.7
The largest randomized elective IABP use trial (BCIS-1) showed a lack of benefit for an elective use of IABP prior to high-risk PCI.1 However, this trial excluded patients in whom a femoral access could not be utilized (ie, like our patient). Additionally, in the BCIS-1 trial, 72% of patient with a maximum BCIS-1 jeopardy score of 12 (like our patient) required a rescue IABP. A rescue IABP in our patient without a femoral access route might have arrived too late! Therefore, we felt it was important to place an IABP electively before PCI.
Our case illustrates two important technical issues which were overcome using relatively simple techniques. First, it demonstrates the feasibility of using an IABP through a brachial approach in tall patients, in whom femoral approach is not feasible. Removal of the protective covering helped lengthen the usable length of the IABP, and it does not affect its overall functionality. Secondly, even if a guide catheter cannot be selectively engaged into the coronary ostium, a guidewire can be used to actually “fetch” the artery and then, using the wire as a support, the guide catheter can be selectively engaged for angiography and support.
References
- Perera D, Stables R, Thomas M, et al. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: A randomized controlled trial. JAMA. 2010;304(8):867-874.
- Cohen M, Urban P, Christenson JT, et al; Benchmark Registry Collaborators. Intra-aortic balloon counterpulsation in US and non-US centres: results from the Benchmark registry. Eur Heart J. 2003;24(19):1763-1770.
- H’Doubler PB Jr, H’Doubler WZ, Bien RC, Jansen DA. A novel technique for intra-aortic balloon pump placement via the left axillary artery in patients awaiting cardiac transplantation. Cardiovasc Surg. 2000;8(6):463-465.
- LaMuraglia GM, Vlahakes GJ, Moncure AC, et al. The safety of intraaortic balloon pump catheter insertion through suprainguinal prosthetic vascular bypass grafts. J Vasc Surg. 1991;13(6):830-835.
- Noel BM, Gleeton O, Barbeau GR. Transbrachial insertion of an intra-aortic balloon pump for complex coronary angioplasty. Catheter Cardiovasc Interv. 2003;60(1):36-39.
- Aznaouridis K, Kacharava AG, Consolini M, Zafari AM, Mavromatis K. Transbrachial intra-aortic balloon pumping for high-risk percutaneous coronary intervention. Am J Med Sci. 2011;341(2):153-156.
- Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44(5):618-623.
____________________________________________
From the University of Oklahoma Health Sciences Center, Cardiovascular Section, Oklahoma City, Oklahoma.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted October 18, 2011, provisional acceptance given October 25, 2011, final version accepted October 27, 2011.
Address for correspondence: Dr Faisal Latif, MD, University of Oklahoma Health Sciences Center, 920 Stanton L Young Blvd., WP #3010, Oklahoma City, OK 73104. Email: latif_faisal@hotmail.com












